Retrospective Comparison of Polymerase Chain Reaction and Culture-Based Identification for Diagnosis of Microsporum canis and Trichophyton Spp. in Shelter Cats and Dogs
DOI:
https://doi.org/10.56771/jsmcah.v3.86Keywords:
ringworm, dogs, cats, shelter medicine, diagnostic methods, Public HealthAbstract
Introduction: Canine and feline dermatophytosis is challenging to manage in shelter settings because of its transmissibility, potential for zoonosis and labour-intensive treatment. An efficient and accurate initial diagnostic plan is essential to confirm infection, prevent outbreaks and ensure public safety. While quantitative polymerase chain reaction (PCR) has gained attention for its rapid turnaround time, studies have shown variable sensitivity. This study aimed to assess the performance of PCR in detecting Microsporum canis and Trichophyton spp. in shelter cats and dogs when compared to culture-based identification.
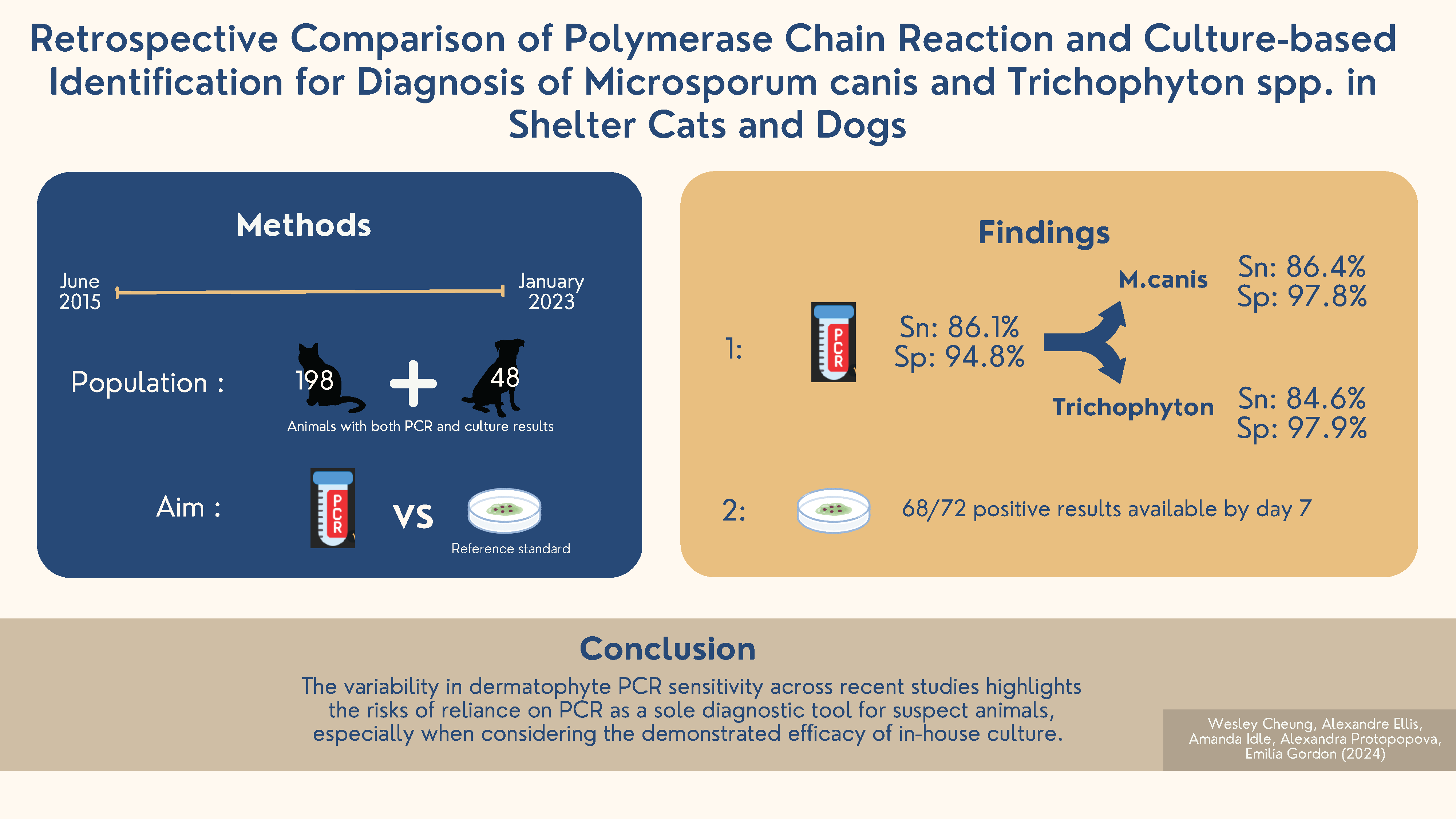
Methods: Between 1 June 2015 and 10 January 2023, 125,939 cats and dogs entered 34 sheltering locations in coastal Western Canada. Of these, 246 animals (48 dogs, 198 cats) had both culture and PCR results, and sufficient records for analysis. Toothbrush samples were collected for dermatophyte test medium (DTM)/enhanced sporulation agar (ESA) bi-plates at a central shelter location and submitted for PCR at a referral laboratory before treatment was initiated. The diagnostic accuracy of PCR was evaluated using the reference standard of a 14-day culture result combined with P-scoring, a semi-quantitative method to assess DTM culture colonies.
Results: Culture readings identified 72/246 (29.27%) lesional animals as positive (63 cats [31.81%] and 9 dogs [18.75%]). PCR demonstrated an overall sensitivity of 86.1% and specificity of 94.8% for both animal species combined. PCR for M. canis showed a sensitivity of 86.4% and a specificity of 97.8%, while PCR for Trichophyton spp. showed a sensitivity of 84.6% and specificity of 97.9%. DTM/ESA culture was highly efficient, with positive results available within 7 days for most cases (59/63 cats [93.6%] and 9/9 dogs [100%]).
Conclusion: The variability in dermatophyte PCR sensitivity across recent studies highlights the risks of reliance on PCR as a sole diagnostic tool for suspect animals, especially when considering the demonstrated efficacy of in-house culture.
Downloads
References
Gordon E, Idle A, DeTar L. Descriptive epidemiology of companion animal dermatophytosis in a Canadian Pacific Northwest animal shelter system. Can Vet J. 2020;61(7):763–770.
Newbury S. Dermatophytosis. In: Miller L, Janeczko S, Hurley KF, eds. Infectious Disease Management in Animal Shelters. 2nd ed. Hoboken, NJ: Wiley-Blackwell; 2021:462–499.
Moriello KA, Coyner K, Paterson S, Mignon B. Diagnosis and treatment of dermatophytosis in dogs and cats: clinical consensus guidelines of the World Association for Veterinary Dermatology. Vet Dermatol. 2017;28(3):266–e68. doi: 10.1111/vde.12440
Fungal Culture Systems. 2014. www.pennvet.com/customer/wcm/connect/352c3217-3c2c-43e9-af4d-7a254a7cfb05/DuoDerm+%26+DuoSab.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-352c3217-3c2c-43e9-af4d-7a254a7cfb05-mqDC.lh. Accessed May 15, 2024.
Newbury S, Moriello K, Verbrugge M, Thomas C. Use of lime sulphur and itraconazole to treat shelter cats naturally infected with Microsporum canis in an annex facility: an open field trial. Vet Dermatol. 2007;18(5):324–331. doi: 10.1111/j.1365-3164.2007.00618.x
Moriello KA, DeBoer DJ. Feline dermatophytosis: recent advances and recommendations for therapy. Vet Clin N M. 1995;25(4):901–921. doi: 10.1016/S0195-5616(95)50134-2
Moriello K. Feline dermatophytosis: aspects pertinent to disease management in single and multiple cat situations. J Feline Med Surg. 2014;16(5):419–431. doi: 10.1177/1098612X14530215
Newbury S, Moriello KA. Feline dermatophytosis: steps for investigation of a suspected shelter outbreak. J Feline Med Surg. 2014;16(5):407–418. doi: 10.1177/1098612X14530213
Stuntebeck R, Moriello KA, Verbrugge M. Evaluation of incubation time for Microsporum canis dermatophyte cultures. J Feline Med Surg. 2018;20(10):997–1000. doi: 10.1177/1098612X17729286
Moriello KA. Treatment of dermatophytosis in dogs and cats: review of published studies. Vet Dermatol. 2004;15(2):99–107. doi: 10.1111/j.1365-3164.2004.00361.x
DeTar LG, Dubrovsky V, Scarlett JM. Descriptive epidemiology and test characteristics of cats diagnosed with Microsporum canis dermatophytosis in a Northwestern US animal shelter. J Feline Med Surg. 2019;21(12):1198–1205. doi: 10.1177/1098612X19825519
Diagnosis and Management of Dermatophytosis with the Ringworm (Dermatophyte) RealPCRTM Panel. IDEXX Laboratories, Inc.; 2017. https://www.idexx.com/files/ringworm-pcr-panel.pdf. Accessed December 12, 2022.
Lee J. IDEXX Laboratories Announces Two New Innovative Tests to Aid Veterinarians in the Diagnosis and Management of Itchy Pets. 2014. https://www.idexx.com/en/about-idexx/news/newsroom-archive/idexx-announces-two-new-innovative-tests-itchy-patients/. Accessed December 12, 2022.
Antech Introduces PCR Ringworm Panel. 2020. https://todaysveterinarybusiness.com/antech-pcr-ringworm-panel/. Accessed December 12, 2022.
Moriello KA, Leutenegger CM. Use of a commercial qPCR assay in 52 high risk shelter cats for disease identification of dermatophytosis and mycological cure. Vet Dermatol. 2018;29(1):66–e26. doi: 10.1111/vde.12485
Jacobson LS, McIntyre L, Mykusz J. Comparison of real-time PCR with fungal culture for the diagnosis of Microsporum canis dermatophytosis in shelter cats: a field study. J Feline Med Surg. 2018;20(2):103–107. doi: 10.1177/1098612X17695899
Dąbrowska I, Dworecka-Kaszak B, Brillowska-Dąbrowska A. The use of a one-step PCR method for the identification of Microsporum canis and Trichophyton mentagrophytes infection of pets. Acta Biochim Pol. 2014;61(2):375–378. doi: 10.18388/abp.2014_1909
Cafarchia C, Gasser RB, Figueredo LA, et al. An improved molecular diagnostic assay for canine and feline dermatophytosis. Med Mycol. 2013;51(2):136–143. doi: 10.3109/13693786.2012.691995
Frost K, Schick A, Mount R. A retrospective analysis of the concordance of in-house fungal culture and a commercial quantitative PCR from 16 dermatology referral practices across the USA (2018–2019). Vet Dermatol. 2022;33(5):392–397. doi: 10.1111/vde.13085
Jacobson LS, McIntyre L, Mykusz J. Assessment of real-time PCR cycle threshold values in Microsporum canis culture-positive and culture-negative cats in an animal shelter: a field study. J Feline Med Surg. 2018;20(2):108–113. doi: 10.1177/1098612X17706270
Katiraee F, Kouchak Kosari Y, Soltani M, Shokri H, Hassan Minooieanhaghighi M. Molecular identification and antifungal susceptibility patterns of dermatophytes isolated from companion animals with clinical symptoms of dermatophytosis. J Vet Res. 2021;65(2):175–182. doi: 10.2478/jvetres-2021-0020
Moskaluk AE, VandeWoude S. Current topics in dermatophyte classification and clinical diagnosis. Pathogens. 2022;11(9):957. doi: 10.3390/pathogens11090957
Saunte DM, Hasselby JP, Brillowska-Dabrowska A, et al. Experimental guinea pig model of dermatophytosis: a simple and useful tool for the evaluation of new diagnostics and antifungals. Med Mycol. 2008;46(4):303–313. doi: 10.1080/13693780801891732
Smith M. Validating real-time polymerase chain reaction (PCR) assays. In: Bamford DH, Zuckerman M, eds. Encyclopedia of Virology. 4th ed. London, UK: Academic Press; 2021:35–44. doi: 10.1016/B978-0-12-814515-9.00053-9
Lewis DT, Foil CS, Hosgood G. Epidemiology and clinical features of dermatophytosis in dogs and cats at Louisiana State University: 1981–1990. Vet Dermatol. 1991;2(2):53–58. doi: 10.1111/j.1365-3164.1991.tb00111.x
Cafarchia C, Romito D, Sasanelli M, Lia R, Capelli G, Otranto D. The epidemiology of canine and feline dermatophytoses in southern Italy. Mycoses. 2004;47(11–12):508–513. doi: 10.1111/j.1439-0507.2004.01055.x
Polak KC, Levy JK, Crawford PC, Leutenegger CM, Moriello KA. Infectious diseases in large-scale cat hoarding investigations. Vet J. 2014;201(2):189–195. doi: 10.1016/j.tvjl.2014.05.020
Moriello KA, Verbrugge MJ, Kesting RA. Effects of temperature variations and light exposure on the time to growth of dermatophytes using six different fungal culture media inoculated with laboratory strains and samples obtained from infected cats. J Feline Med Surg. 2010;12(12):988–990. doi: 10.1016/j.jfms.2010.07.019
Akhoundi M, Nasrallah J, Marteau A, Chebbah D, Izri A, Brun S. Effect of household laundering, heat drying, and freezing on the survival of dermatophyte conidia. J Fungi (Basel). 2022;8(5):546. doi: 10.3390/jof8050546
Sparkes AH, Werrett G, Stokes CR, Gruffydd-Jones TJ. Microsporum canis: inapparent carriage by cats and the viability of arthrospores. J Small Anim Pract. 1994;35(8):397–401. doi: 10.1111/j.1748-5827.1994.tb03861.x
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Wesley Cheung, Alexandre Ellis, Amanda Idle, Alexandra Protopopova, Emilia Gordon

This work is licensed under a Creative Commons Attribution 4.0 International License.








