Treatment and Outcomes of Sheltered Cats with Feline Panleukopenia using Canine Parvovirus Monoclonal Antibody (CPMA) [Abstract]
DOI:
https://doi.org/10.56771/jsmcah.v4.132Abstract
Introduction
Feline panleukopenia virus (FPV) remains a serious threat to shelter cat health and welfare, with mortality in kittens as high as 90%.1 Treatment with immunoglobulins and/or hyperimmune serum has been described previously as having the therapeutic effect of canine immunoglobulin fragments in FPV-infected cats.2 Canine parvovirus monoclonal antibody (CPMA) is commercially available for intravenous use in dogs to treat canine parvovirus; however, no peer-reviewed data are available for its use in cats. For these reasons, Charleston Animal Society adopted the use of CPMA in cats as an experimental treatment with hopes that it would improve live outcomes and shorten the treatment course for panleukopenia.
Methods
Charleston Animal Society is a private, government-contracted, open-intake shelter with a 2024 intake of 9,877 dogs and cats. In 2024, feline intake was 6,766 with a non-live outcome of 7.6%. Charleston Animal Society routinely treats kittens and cats for panleukopenia. Cats with a positive point-of-care canine parvovirus antigen test result (Snap Parvovirus, IDEXX) in combination with clinical signs (vomiting, diarrhea, lethargy) were diagnosed with panleukopenia by a veterinarian or licensed veterinary technician. Standard treatment included antibiotics (oral or parenteral), fluids (subcutaneous or intravenous), anti-emetics, and treatment for diarrhea. Other symptomatic treatments were administered as needed based on the patient’s exam. CPMA was added to the standard treatment protocol midway through 2024; it was given subcutaneously at 0.2ml/kg based on anecdotal reports from other shelter veterinarians. Operational and clinical outcomes for cats treated prior to and after the inclusion of CPMA were compared.
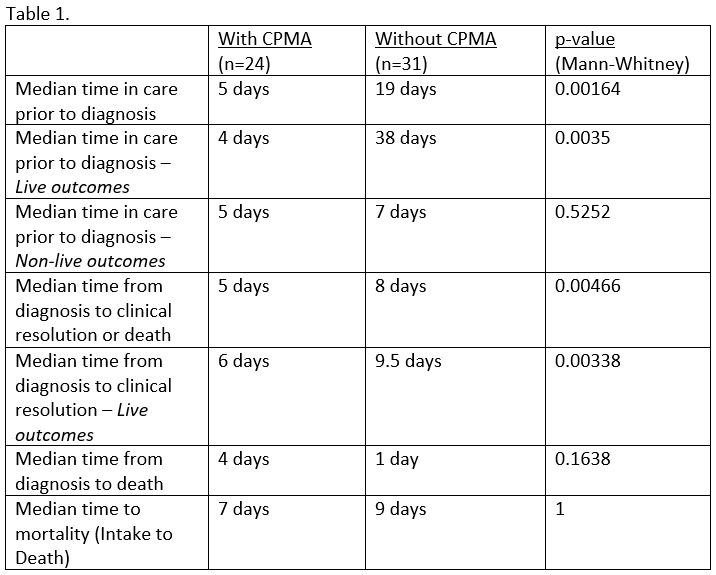
Results
Fifty-five cats were diagnosed with and treated for panleukopenia; 31 cats were treated prior to the adoption of CPMA, and 24 were treated with a protocol that included CPMA (Table 1). Median age at diagnosis (2 months) and median age of mortality (1 month) were the same for both groups. Median time to clinical resolution and treatment discontinuation was 3.5 days shorter for the CPMA-treated group. Overall survival rate was 85.5%; there was no statistically significant difference (Fisher exact, p=0.270) in mortality rate between those treated with CPMA (20.8%, n=5) and those without CPMA (9.7%, n=3). Overall, no adverse effects were attributed to the administration or use of CPMA.
Conclusion
The use of CPMA in the treatment of panleukopenia, in conjunction with a full protocol of supportive care, can be considered. No evident negative outcomes were noted in the CPMA-treated kittens, and the treatment course for these patients was approximately 3 days shorter. For shelters looking to decrease the length of stay in isolation to treat panleukopenia, CPMA may be helpful.
Financial Support and Conflict of Interests BD is a consultant for Elanco Animal Health.
Downloads
References
1. Truyen, U., Addie, D., Belak. S. et al. Feline panleukopenia ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery 2009; 11 (7): 538-546. http://dx.doi.org/10.1016/j.jfms.2009.05.002
2. Pei, Z., Sun, M., and Zhang, X., et al. Canine immunoglobulin F(ab’)2 fragments protect cats against feline parvovirus infection. International Immunopharmacology 2020 86 (10672) https://doi.org/10.1016/j.intimp.2020.106752
Published
Issue
Section
License
Copyright (c) 2025 The Authors

This work is licensed under a Creative Commons Attribution 4.0 International License.










