THE ASSOCIATION OF SHELTER VETERINARIANS’ GUIDELINES FOR STANDARDS OF CARE IN ANIMAL SHELTERS: Second Edition - December 2022
Lena DeTar* DVM, MS, DACVPM, DABVP (Shelter Medicine Practice) Maddie’s Shelter Medicine Program, Cornell University College of Veterinary Medicine, Ithaca, NY
Erin Doyle* DVM, DABVP (Shelter Medicine Practice) American Society for the Prevention of Cruelty to Animals, Boston, MA
Jeanette O’Quin* DVM, MPH, DACVPM, DABVP (Shelter Medicine Practice) The Ohio State University College of Veterinary Medicine, Columbus, OH
Chumkee Aziz DVM, DABVP (Shelter Medicine Practice) University of California-Davis, Koret Shelter Medicine Program, Houston, TX
Elizabeth Berliner DVM, DABVP (Shelter Medicine; Canine & Feline Practice) American Society for the Prevention of Cruelty to Animals, Ithaca, NY
Nancy Bradley-Siemens DVM, MNM, MS, DABVP (Shelter Medicine Practice) Shelter and Community Medicine, Midwestern University, College of Veterinary Medicine, Glendale, AZ
Philip Bushby DVM, MS, DACVS Shelter Medicine, College of Veterinary Medicine, Mississippi State University, Starkville, MS
Staci Cannon DVM, MPH, DACVPM, DABVP (Shelter Medicine Practice) University of Georgia College of Veterinary Medicine, Athens, GA
Brian DiGangi DVM, MS, DABVP (Canine & Feline Practice; Shelter Medicine Practice) University of Florida College of Veterinary Medicine, Gainesville, FL
Uri Donnett DVM, MS, DABVP (Shelter Medicine Practice) Dane County Humane Society, Madison, WI
Elizabeth Fuller DVM Charleston Animal Society, Charleston, SC
Elise Gingrich DVM, MPH, MS, DACVPM, DABVP (Shelter Medicine Practice) American Society for the Prevention of Cruelty to Animals, Fort Collins, CO
Brenda Griffin, DVM, MS, DACVIM (SAIM), DABVP (Shelter Medicine Practice) University of Florida College of Veterinary Medicine, Gainesville, FL
Stephanie Janeczko DVM, MS, DABVP (Canine & Feline Practice; Shelter Medicine Practice), CAWA, American Society for the Prevention of Cruelty to Animals, New York, NY
Cristie Kamiya DVM, MBA, CAWA Humane Society Silicon Valley, Milpitas, CA
Cynthia Karsten DVM, DABVP (Shelter Medicine Practice) University of California-Davis, Koret Shelter Medicine Program, Sacramento, CA
Sheila Segurson, DVM, DACVB Maddie’s Fund, Pleasanton, CA
Martha Smith-Blackmore DVM Forensic Veterinary Investigations, LLC, Boston, MA
Miranda Spindel DVM, MS Shelter Medicine Help, Fort Collins, CO
*Editors
Citation: Journal of Shelter Medicine and Community Animal Health 2022 - http://dx.doi.org/10.56771/ASVguidelines.2022
Copyright: © 2022. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Published: 23 January 2023
Acknowledgements
The ASV would like to recognize and thank the authors of the first edition of the ASV Guidelines for Standards of Care in Animal Shelters for their time and dedication to creating and sharing this transformative document: Sandra Newbury, Mary Blinn, Philip Bushby, Cynthia Barker Cox, Julie Dinnage, Brenda Griffin, Kate Hurley, Natalie Isaza, Wes Jones, Lila Miller, Jeanette O’Quin, Gary Patronek, Martha Smith-Blackmore, Miranda Spindel.
We would like to acknowledge the following people for their assistance with the presentation of this document:
- Dr. Denae Wagner for drafting figures of dog and cat primary enclosure set-up
- Katie Mihalenko for graphic design
- Gene Summerlin for legal consultation
- Abigail Appleton, PMP, CAWA, for technical support creating the checklist of key actionable statements
- Open Academia for publishing services
Introduction
Purpose
The Association of Shelter Veterinarians’ (ASV) Guidelines for Standards of Care in Animal Shelters [‘The Guidelines1’] was originally published in 2010. While animal sheltering has evolved substantially in the last decade, this second edition shares the same fundamental goals. To provide:
- a set of common standards for the care and welfare of companion animals in shelters based on scientific evidence and expert consensus
- guidance that helps animal welfare organizations reduce overcrowding, stress, disease, and improve safety
- a tool for animal welfare organizations and communities to assess and improve their shelters
- references for creating regulations and statutes around sheltering, and benchmarks for organizational change
- guidance for animal housing in existing facilities and priorities for the design of new construction
- a living document that responds to developments in shelter medicine and animal care research and practice
Both documents share the guiding principle that meeting each animal’s physical and emotional needs is the fundamental obligation of a shelter regardless of the mission of the organization or the challenges involved in meeting those needs.
About this document
This second edition keeps the intent and format of the original document, while incorporating important updates based on the growing body of animal sheltering science and recommendations rooted in practical experience. To undertake this revision, the Board of Directors of the ASV formed a task force of 19 shelter veterinarians from a pool of nominees and original authors. Task force members were selected from those active within the ASV community to provide diversity and breadth in their areas of expertise, geographical locations, and current or previous roles in a variety of shelter types. Task force members completed literature reviews and consulted subject matter experts to inform their contributions. Funding to support the research, development, and publication of this document was provided by the ASV. No commercial or industry funding was used.
This consensus document, which represents the collective input and agreement of all task force members, took 3 years to create. This second edition was approved unanimously by the ASV Board of Directors in December 2022.
Audience
The Guidelines for Standards of Care in Animal Shelters, Second Edition, is written for organizations of any size or type who provide temporary housing for companion animals. The term shelter used here includes foster-based rescues, nonprofit humane societies and SPCAs, municipal animal services facilities, and hybrid organizations. The Guidelines are also applicable to any organization that routinely cares for populations of companion animals, including companion animal sanctuaries, cat cafés, vet clinics, pet stores, dog breeding operations, research facilities (including universities), and service, military, or sporting dog organizations. This document was written for organizations working in every community, including those with significant numbers of homeless pets, those with the capacity to take in animals from other locations, and those whose pet population challenges vary by species, time of year, and other circumstances.
The term personnel is used in this document to include all paid and volunteer team members caring for animals in shelters and foster-based organizations. This document is intended to guide all personnel, including administrative, medical, behavior, and animal care staff; volunteers; foster caregivers; sole operators; and those filling any other role that supports animal well-being.
Scope
Although many practice recommendations and examples are included, these Guidelines are not a detailed manual for shelter operations. As with the previous document, the aim is to provide guiding standards of care to meet animals’ needs, while allowing shelters to determine exactly how those standards are met in their own operating protocols, based on their mission or mandate, resources, challenges, and community needs.
In this document, we have deliberately limited our focus to the care of cats and dogs who make up the majority of animals admitted to shelters in the United States every year. When caring for other species, similar operational principles can be applied to meet the unique needs of those animals.
The ASV recognizes the importance of activities supporting pet retention and access to veterinary care, and that shelters are playing a large role in providing those services.2 Informed community engagement is critical in supporting the health of animals in their communities, with impacts on shelter intake and human health.3 Although these services are addressed where they intersect with shelter admission and outcome policies and decisions, this document does not focus specifically on how shelters support owned animals or community pet welfare.
Format
These Guidelines have been divided into 13 sections; 11 have been updated from the original document and two are new. The document is intended to be read in its entirety because concepts build upon one another. A glossary is included as Appendix A; a checklist of key actionable statements is available on the ASV website. Lists of helpful resources are also included in appendices for ease of access. As an evidence-based document, the many references included direct the reader to the science and research behind specific recommendations.
As with the original document, the key actionable statements use an unacceptable, must, should, or ideal format:
- Unacceptable indicates practices that need to be avoided or prevented without exception
- Must indicates practices for which adherence is necessary to ensure humane care
- Should indicates practices that are strongly recommended, and compliance is expected in most circumstances
- Ideal indicates practices that are implemented when resources allow
The ASV recognizes that each organization is uniquely situated and faces challenges that may impact their ability to implement the practices recommended. The ranked format of statements allows organizations to set priorities for improving their operations and facilities. This is not a legal document; shelters should be aware that state and local laws and regulations may supersede the recommendations made here.
Ethical framework for animal welfare
The ethical principles for animal welfare used in the original Guidelines document were the Five Freedoms: the freedom from hunger and thirst; the freedom from discomfort; the freedom from pain, injury, or disease; the freedom to express normal behavior; and the freedom from fear and distress.1,4
While these principles are valuable for defining essential elements of animal welfare, their focus is on avoiding negative experiences. Positive experiences and welfare are also essential to promote a life worth living.5 For example, shelters do more than ensure animals do not go hungry; they regularly provide species- and life stage-specific food that nourishes, provides interest, and satisfies without overfilling. Food can be even more enriching when provided in a context of social contact and animal training.
The Five Domains model, derived from the Five Freedoms, illustrates how better or worse nutrition, environment, physical health, and behavioral opportunities combine to inform an animal’s mental state, which, in turn, informs their overall welfare.6 This model does two new things. First, it gives a spectrum for each domain, for example, allowing not just the absence of pain but including the feelings of comfort and fitness (Table 1).
| 1. Nutrition | 2. Environment | 3. Health | 4. Opportunity | 5. Mental state | |
| Positive experiences | Enough food and water Fresh clean water Balanced, variety of food |
Comfortable Temperate Routine Clean Interest/variety |
Physical health Good function Good body condition Restful sleep |
Choice of environment Choice of interaction Behavioral variety (play, hunt, forage, engage, rest) Novelty |
Satisfied Engaged Comfortable Affectionate Playful Confident Calm Encouraged |
| Negative experiences | Restricted water Restricted food Poor quality Monotonous |
Too cold or hot Too dark or bright Too loud or quiet Unpredictable Malodorous Soiled Monotonous Uncomfortable |
Body dysfunction or impairment Disease Pain Poor fitness |
Barren cage Confined space Separation from people or species Restraint Unavoidable sensory inputs |
Fearful or anxious Frustrated Bored, lonely Exhausted Ill, painful Uncomfortable Hungry, thirsty |
| Adapted from Mellor6 | |||||
Second, this model illustrates that positive welfare states can still occur even when one or more important needs are not completely satisfied. For example, a stray cat with a healing pelvic fracture on cage rest (restricted agency, pain) may still have an overall positive welfare state when appropriately treated and housed in an enriched foster home. Negative mental states are also possible even if only one need is unmet. For example, a well-fed and physically healthy dog confined long-term to a kennel (restricted agency) may have profound mental distress and overall negative welfare.
When nutritional, environmental, physical, and emotional needs are increasingly satisfied, animals have increasingly positive mental states and demonstrate this through physical manifestations of good health and behavior (Figure 1).
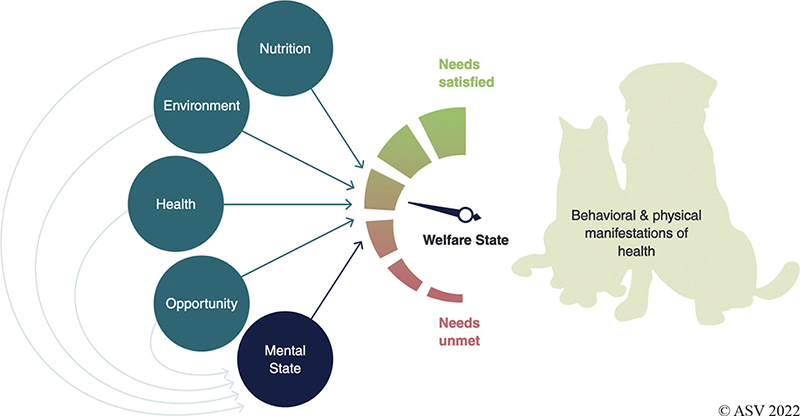
Figure 1. The Five Domains of animal welfare in action
In this document, we set out to help shelters achieve positive welfare in each of these Five Domains within the necessary constraints of animal and human safety and infectious disease control. In addition to following the Guidelines in this document, we hope that shelters will examine existing practices in light of the Five Domains framework and identify new ways to tip the balance toward positive well-being for the animals in their care.
Sheltering today
This document was created during a period of social upheaval, with a global pandemic, climate events, and racial inequity protests impacting communities around the world. Both the COVID-19 pandemic and increasingly frequent damaging weather events have accentuated the critical role that shelters play in keeping animals safe and preserving the human–animal bond. The willingness of communities to help shelters was also highlighted during the pandemic, when entire organizations pivoted to foster care and pursued creative alternatives to intake. Inviting members of the community to be a part of the safety-net has created opportunities for new programs and bigger impacts.
At the same time, the animal welfare industry has been reflecting on how sheltering and animal control practices contribute to systemic inequities in their communities, including the ways that shelters admit, transport, and adopt out animals. This reflection has emphasized the need for accessible, non-punitive services for pet owners in our communities, the benefits of culturally sensitive community engagement, and the need to work toward representing the diversity of our communities in our personnel and profession (ASV’s Commitment to Diversity, Equity and Inclusion).7 Staffing and work environment challenges, during the pandemic and beyond, have reiterated the need for shelters to be healthy, supportive, and inclusive places to work and volunteer (ASV’s Well-being of Shelter Veterinarians and Staff).8
Confronting these challenges together has created a stronger, more interconnected animal welfare community. The ASV offers this document as a tool to help shelters connect to expert guidance and measure themselves against a common standard, to help personnel find compassion satisfaction, to solidify the shelter’s role in supporting their community, and to elevate the welfare of animals in their care.
References
| 1. | Newbury S, Blinn MK, Bushby PA, et al. Guidelines for Standards of Care in Animal Shelters. The Association of Shelter Veterinarians; 2010:1–67. |
| 2. | Shelter Animals Count. Community Services Data Matrix. 2021:1–10. Accessed Dec 13, 2022. https://shelteranimalscount-cms-production.s3.us-east-2.amazonaws.com/sac_communityservicesdatamatrix_202101_c1ddc2b4b6.pdf |
| 3. | Clinical and Translational Science Awards Consortium Community Engagement Key Function Committee Task Force on the Principles of Community Engagement. Prinicples of Community Engagement. 2nd ed. Silberberg M, Cook J, Drescher C, McCloskey DJ, Weaver S, Ziegahn L, eds. National Insitutes of Health and Human Services; 2011. |
| 4. | Elischer M. The Five Freedoms: A History Lesson in Animal Care and Welfare. Michigan State University Extension; 2019. Accessed Dec 13, 2022. https://www.canr.msu.edu/news/an_animal_welfare_history_lesson_on_the_five_freedoms |
| 5. | Mellor DJ. Animal emotions, behaviour and the promotion of positive welfare states. N Z Vet J. 2012;60(1):1–8. doi: http://dx.doi.org/10.1080/00480169.2011.619047. |
| 6. | Mellor DJ. Updating animalwelfare thinking: moving beyond the “five freedoms” towards “A lifeworth living.” Animals. 2016;6(3):21. doi: 10.3390/ani6030021 |
| 7. | Association of Shelter Veterinarians. ASV’s Commitment to Diversity, Equity, and Inclusion. 2020. |
| 8. | Association of Shelter Veterinarians. Position Statement: Well-being of Shelter Veterinarians and Staff. 2022. |
1. Management and Record Keeping
1.1 General
A well-run sheltering organization of any size is built on a foundation of planning, training, and oversight. This foundation is an essential part of implementing the guidelines presented in this document. Shelters must have a clearly defined mission or mandate, adequate personnel, up-to-date policies and protocols, a system for training and supervising personnel, and management practices aligned with these guidelines.
The shelter’s mission or mandate should reflect the needs of the community it serves. Tools that aid shelters in defining their purpose include community needs assessments and strategic planning. A community needs assessment reveals what services are already being provided in the community and where needs are unmet. Programs and collaborations have the biggest impact when they reflect principles of community engagement, including respect for each other’s values and cultures.1 The community’s needs should be regularly reviewed, and strategies and goals updated accordingly.
Strategic planning is an organizational process used to define the shelter’s essential programs and goals, and then purposefully allocate resources (e.g. shelter space, personnel, and finances) toward achieving these goals. This planning positively impacts an organization’s ability to achieve its stated objectives.2 Strategic plans are most effective when reviewed regularly, often quarterly, to ensure progress is being made and goals are still relevant.
Animal shelter administration requires the balance of a complex set of considerations, including a focus on collaboration and the establishment of best practices. When developing organizational level policies and protocols, administrators are encouraged to consult industry-specific professional organizations for guidance and to learn from the experience of others in the field.3–5 Because animal health and welfare is woven into every facet of shelter operations, veterinarians should be integrally involved with development and implementation of the shelter’s organizational policies and protocols.
1.2 Management structure
Shelters must have a clearly defined organizational structure that outlines accountability, responsibility, and authority for management decisions. This organizational structure must be communicated to all staff and volunteers. Organizational charts are visual tools that enable all personnel to understand roles and responsibilities, supporting clear communication across departments. This blueprint of the organization can be used by new team members learning about the organization, by those in leadership planning for growth and transition, and by external partners establishing a collaborative relationship with the organization. Lines of authority, responsibility, and supervision should be in writing, reviewed periodically, and updated when roles change.
Decision-making must take into account resource allocation as well as population and individual animal health and welfare. Decisions involving the allocation of resources, whether at the organizational, population, or individual animal level, are best made by personnel aware of organizational priorities and the shelter’s capacity for care.
Authority and responsibility for tasks and decision-making must be given only to those who have the appropriate knowledge, training, and when applicable, credentials. For example, resource-based decisions (e.g. to treat or to euthanize an individual animal) may be made by shelter personnel, but medical treatment decisions (e.g. which drug to treat with) need to involve a veterinarian.
The practice of veterinary medicine and surgery is restricted to those with a valid license. In the United States, veterinary practice is defined by state or territorial practice acts. These acts generally cover the diagnosis and treatment of medical conditions, prescription of pharmaceuticals, surgery, and the tasks that other personnel (e.g. technicians, assistants, veterinary students, and others) may perform under direct or indirect veterinary supervision.6 Several states and the AVMA Model Veterinary Practice Act have sections specific to population medicine and the provision of veterinary oversight through standard written protocols and timely visits to the premises where animals are housed.7,8
Some medical procedures (e.g. microchipping and alternative therapies) may be restricted to veterinarians in some states and not in others.9 Shelters can maximize capacity for medical services by using veterinary technicians and other veterinary professionals to the extent of their capabilities. Providing veterinary care via telemedicine extends veterinary bandwidth and can improve animal welfare.10
A formal relationship with a veterinarian must be in place to ensure oversight of medical and surgical care in the shelter. Many shelters employ one or more veterinarians, others may use local veterinary clinics, and some use paid or unpaid contract veterinarians. A shelter’s veterinarian must have knowledge about their particular population and should have training or experience in shelter medicine. The shelter’s veterinarian should be consulted on all policies and protocols related to the maintenance of medical and behavioral animal health (see Medical Health). Furthermore, veterinarians may be uniquely suited to provide training and continuing education, communicate with external stakeholders, and engage in organizational policy and protocol development in shelters.
1.3 Establishment of policies and protocols
Organizational policies are a framework of high-level decisions that ensure operations remain consistent with the shelter’s mission and priorities. Shelter policies help ensure that animal needs do not overwhelm the resources available to meet those needs, since operating beyond an organization’s capacity for care is unacceptable (see Population Management). Important policies for sheltering organizations include intake, treatable conditions, euthanasia, adoption, transport, and community animal services.
Shelter protocols are critical tools that ensure consistent daily operations in keeping with organizational policies. Protocols must be developed and documented in sufficient detail to achieve and maintain the standards described in this document and should be reviewed and updated regularly. All personnel must have access to up-to-date protocols. How shelters provide this access will vary by organization and may include digital or paper documents. Shelter management must routinely monitor and ensure compliance with protocols. Appendix B provides a comprehensive list of protocols recommended in these Guidelines.
Shelters are obligated to comply with all local, state, and national regulations, which need to be reviewed regularly. In some cases, existing regulations may represent outdated practice or lower standards of care and can restrict or even conflict with current best practices. When implementation of these Guidelines does not align with government regulations or policies, shelters are encouraged to support endeavors for legislative change.
1.4 Training
Effective training of personnel (i.e. paid and unpaid staff and volunteers) is necessary to ensure safe and humane animal care and the safety of people.11 Personnel training should incorporate all relevant aspects of working in the organization. In addition to operating protocols for daily tasks, effective training programs include broader topics that help staff to perform their duties well, such as communication techniques; data management; animal husbandry; staff well-being; and diversity, equity, and inclusion (Appendix B).
Onboarding is an important part of introducing new personnel to any organization. Shelters must provide training for each shelter task, and personnel must demonstrate skills and knowledge before proficiency is assumed. For example, new animal care staff could complete virtual training materials on sanitation and work with a senior staff member prior to being assigned to sanitize enclosures.
Documentation of training should be maintained and reviewed regularly as a part of professional development and performance reviews. Ongoing feedback about performance, both in-the-moment and through formal reviews, is an important element of professional growth for personnel at all levels. When licensing or certification is required to perform specialized duties, as in veterinary care or euthanasia, personnel performing these tasks must be credentialed.12,13 Continuing education must be provided for all personnel in order to improve skills and maintain credentials. Investing in training requires time and resources but is key to program success.
To ensure employee, volunteer, and public safety, shelters must provide all personnel the information and training needed to recognize and protect themselves against common zoonotic conditions (see Public Health). In addition, shelter personnel having any form of contact with animals should have proper training in basic animal handling skills, animal body language, and bite prevention strategies. This training reduces risk for staff and volunteers and provides a more humane experience for animals.
1.5 Record keeping and animal identification
Shelter animal identification and maintenance of animal records are essential for shelter operations. Shelters must adhere to the elements of record-keeping defined within regulatory requirements.
Given the wide availability of technology, digital systems should be used for record keeping, preferably software systems designed for animal shelters. With proper utilization, shelter software or spreadsheet programs allow organizations to better manage resources, schedules, and shelter processes. The software system used by a shelter should be able to generate basic population level reports as well as individual animal records. Population-level data inform management strategies (see Population Management) and allow regular assessment and reporting of organizational goals and activities.14
No matter the system used, each animal must have a unique identifier and individual record. This identifier (e.g. name and number) is established at or prior to admission and ensures consistency and accuracy in care and record keeping for that animal. Shelter software programs typically generate a ‘kennel card’ based on animal information entered into the system, which can be displayed on or near the animal’s primary enclosure for easy reference by personnel and the public.
Because animals may move within and between areas, shelters must have an organized system by which animal identification information can be quickly and easily matched to animals in enclosures and their shelter records. Since identification may be challenging when animals are outside of their enclosures, co-housed with similar animals, or in foster homes, a means of identification should be physically affixed (e.g. collar and tag) or permanently inserted (microchip), when it is safe to do so.
Shelter records should capture all pertinent medical and behavioral information (Table 1.1.) Records must be maintained for animals in foster care and other off-site housing locations just as they are for shelter-housed animals.
References
| 1. | Clinical and Translational Science Awards Consortium Community Engagement Key Function Committee Task Force on the Principles of Community Engagement. Prinicples of Community Engagement. In: Silberberg M, Cook J, Drescher C, McCloskey DJ, Weaver S, Ziegahn L, eds. 2nd ed. National Insitutes of Health and Human Services; 2011, pages 1–188. |
| 2. | George B, Walker RM, Monster J. Does Strategic Planning Improve Organizational Performance? A Meta-Analysis. Public Adm Rev. 2019;79(6):810–819. doi: 10.1111/PUAR.13104 |
| 3. | Association of Animal Welfare Administrators. Resources. Accessed Dec 13, 2022. https://theaawa.org/page/Resources. |
| 4. | National Animal Care and Control Association. Home: National Animal Care & Control Association. Accessed Dec 13, 2022. www.naca.com |
| 5. | Association of Shelter Veterinarians. Association of Shelter Veterinaians: Home. Accessed Dec 13, 2022. www.sheltervet.org |
| 6. | Association of Shelter Veterinarians. Position Statement: Veterinary Supervision in Animal Shelters. 2021;1. Accessed Dec 13, 2022. https://www.sheltervet.org/assets/docs/position-statements/VeterinarySupervision in Animal Shelters PS 2021.pdf. |
| 7. | American Veterinary Medical Association, AVMA. AVMA Policy: Model Veterinary Practice Act. J Am Vet Med Assoc. 2021. Accessed Dec 13, 2022. https://www.avma.org/sites/default/files/2021-01/model-veterinary-practice-act.pdf. |
| 8. | American Association of Veterinary State Boards. Veterinary Medicine and Veterinary Technology Practice Act Model (PAM). 2019. Accessed Dec 13, 2022. https://www.aavsb.org/board-services/member-board-resources/practice-act-model/. |
| 9. | American Veterinary Medical Association. Policy: Complementary, Alternative, and Integrative Veterinary Medicine, Shaumburg IL, 2022. |
| 10. | Association of Shelter Veterinarians. ASV Telemedicine Position Statement. Accessed Dec 13, 2022. https://www.sheltervet.org/assets/docs/position-statements/Telemedicine PS 2021.pdf. |
| 11. | National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (U.S.). Guide for the Care and Use of Laboratory Animals. National Academies Press; 2011, Washington DC. |
| 12. | American Association of Veterinary State Boards. Licensing Boards for Veterinary Medicine, Shaumburg IL. |
| 13. | American Veterinary Medical Association. State Laws Governing Euthanasia. 2022. Accessed Dec 13, 2022. https://www.avma.org/advocacy/state-and-local-advocacy/state-laws-governing-euthanasia. |
| 14. | Shelter Animals Count. Basic Data Matrix. Accessed Dec 13, 2022. https://www.shelteranimalscount.org/wp-content/uploads/2022/02/BasicDataMatrix_SAC.pdf. |
2. Population Management
2.1 General
Shelters must practice active population management, which is the process of intentionally and efficiently planning services for each animal in the shelter’s care. Individual animals are managed in consideration of the shelter’s ability to care for that animal and their entire population in a manner consistent with the guidelines outlined in this document. Population management includes pre-intake planning, protocols for care and services, ongoing daily evaluation, outcome planning, and response to changing conditions of the shelter and the animal.1
Every organization has limits to its ability to provide care. Limits include financial and physical resources, personnel hours and skills, housing and operations space, and the opportunity for live outcomes. These limitations define the number and type of animals for which an organization can provide humane care, also known as the organization’s capacity for care. The concept of capacity for care is not unique to animal sheltering and is recognized in veterinary hospitals, other animal care fields, human healthcare, hospitality, and other industries.2,3
Operating beyond an organization’s capacity for care is an unacceptable practice. When shelter populations tax the organization’s ability to provide care for their animals, living conditions worsen, and population health and well-being are compromised.4,5 Delays in recognizing problems and providing services negatively impact animal welfare and prolong the length of stay (LOS) for animals in shelters. Alternatively, working to maintain the population within the shelter’s capacity for care has been linked to decreased LOS, decreased disease and euthanasia rates, and increased live outcomes.6,7 Policies and protocols must be in place to ensure an organization operates within its capacity for care.
2.2 Determining capacity for care
The most visible factor in determining the shelter’s capacity for care is housing capacity, or the number of available humane housing units. Housing units include in-shelter enclosures as well as foster homes and off-site housing. Housing capacity calculations must be based on the ability to promote each animal’s positive welfare. Housing units that are too small or otherwise inappropriate cannot be included (see Facilities). The number of humane housing units available may exceed an organization’s capacity for care, since the organization’s capacity is also determined by shelter personnel, resources, and available outcomes.
The time and skills of shelter personnel is another critical component of a shelter’s capacity for care. Trained personnel must be scheduled to meet daily animal care needs and efficiently and effectively accomplish each critical task. A standard estimate such as 15 minutes per animal per day8 may roughly calculate the time needed for cleaning and feeding in some facilities, but it does not account for variations in housing designs and sanitation protocols, the time needed for training personnel, and the provision of enrichment and additional care.9 Personnel time needed for essential care tasks such as sanitation, feeding, and enrichment is best estimated using direct observation to calculate the average time per task. These estimates, when multiplied by the number of animals in care, can guide staffing levels and schedules. Direct observation is also useful for estimating the time needed for personnel to complete other critical tasks, such as intake, rounds, assessments, and outcome processes.
Animals with medical and behavioral challenges may need more care time per day and may also require services from personnel with advanced skills or credentials. When these services are provided by external partners, a shelter’s capacity for care will also be affected by the capacity of these partners. Services such as surgery, veterinary visits, or transport should be scheduled in anticipation of an animal’s eligibility for that service. Proactive scheduling can maximize the use of external partner capacity.
Foster programs must have sufficient personnel to provide support to caregivers and animals. Foster support includes tasks such as maintaining a foster caregiver database, communicating with foster caregivers, scheduling appointments, and facilitating outcomes. Medical, surgical, and behavioral services for foster animals must be provided in a manner that promotes animal welfare and minimizes LOS.
Shelter resources, including finances and material goods, are another critical factor in determining an organization’s capacity for care. If a shelter cannot afford or otherwise procure supplies or necessary services for the animals in their facility, animal welfare will be compromised. There is no standard estimate for calculating cost of care per animal but using historical organizational information and comparing budgets with similar organizations can help shelters manage their available resources.
Shelters should engage with one another to leverage resources and maximize each organization’s strengths. Thoughtful partnerships avoid redundancy and increase the community’s capacity to help animals. For example, a small organization with limited medical resources can partner with a larger organization with a full-service hospital, or a brick-and-mortar organization can partner with a foster-based organization to house animals with kennel-induced stress. In addition to partnering with other animal welfare organizations, collaborating with human service professionals, such as social workers, housing advocates, and home care providers, can support pet retention and prevent relinquishment.
2.3 Operating within capacity for care
Shelters experience a high demand for their services. Working within their capacity for care maximizes each shelter’s impact through thoughtful planning and efficient decision-making. An organization’s policies for admissions and outcomes should be based on their mandate, mission, and the needs of their community. When organizations find that they are frequently near or over their capacity for care, strategic planning can be a valuable process to address how a shelter’s capacity for care and their community’s needs can better align (see Management and Record Keeping).
2.3.1 Admission planning
When appropriate, admission policies should prioritize retention over shelter intake. Helping pets stay with their owner or caregiver preserves the human–animal bond, eliminates the stress of shelter admission, and addresses discriminatory admissions practices.10 Owners may be able to keep their pet if given access to services, supplies, or information.11
Decisions about intake must consider whether admission is the best option for the animal or their situation. Gathering and providing information prior to admission can support intake diversion. For example, finders can be provided information about neonatal kitten care, so that they can rear kittens in their home until they are old enough to be adopted.
Admission must be balanced with the ability to provide appropriate outcomes, minimize LOS, and ensure the shelter remains within its capacity for care. Population management begins prior to admission: an animal must only be admitted if the shelter can provide the care they require. For welfare or safety reasons, some animals may need to be admitted so that euthanasia can be provided.
When admission is deemed the best solution for an animal, situation, and shelter, appropriate intake scheduling ensures that the shelter has the capacity to care for this animal and the animals already in care.12,13 Intake by appointment is recommended even for shelters with high intake demand and open admissions policies and can be used to control the flow of animals into the shelter.11,13,14
Organizations that are impacted by unpredicted intakes (e.g. disasters and large-scale investigations) must have a plan to flex their operations to increase their capacity for care. Compromising the welfare of animals and personnel is not an acceptable strategy for meeting the increased care demands of unpredicted intakes. Increasing a shelter’s capacity requires more than identifying additional humane housing units; all aspects of care need to flex to match, including increased animal care personnel and hours, medical and behavioral care services and providers, resources to supply and fund the response, and a range of available outcomes.15
2.3.2 Outcome planning
Every attempt must be made to locate a lost animal’s owner, including careful screening for identification and microchips, in the field and at the time of intake. Field agents and admissions personnel require ready access to lost pet data and social media in order to cross-check identifying features of animals being picked-up or brought in. Lost pets are usually found close to home and may be returned to their owner without shelter admission.16,17 Reunification of pets can be an opportunity to provide owners with services or information promoting identification (microchipping and ID tags), spay-neuter, training, or fence-building programs. Shelters can also support community members working to reunite animals with their owners directly.
In addition to prioritizing pet retention and reunification, shelters should remove barriers to local outcomes. Removing barriers can include:
- accessible and convenient open hours
- adoption and reclaim services in languages spoken by the community
- affordable adoption and reclaim fees
- adoption and outreach events that reach the entire community
- inclusive adoption policies
Imposing strict policies or requirements on adopters (e.g. employment status, landlord checks, home visits, and veterinary references) is discriminatory, prolongs LOS in the shelter, and prevents future adoptions.18 Strategies that support pet retention, reunification, and local adoption acknowledge the community’s ability and desire to provide care for their pets.
Relocation of animals for adoption can be a valuable strategy for live outcomes while working to address population challenges and remove barriers to local outcomes (see Animal Relocation and Transport). Destination shelters need to critically consider their capacity for care before making the decision to take in transported animals. These programs are not a replacement for partnership building within the local community.
2.3.3 Length of stay
The number of animals a shelter has in its care on any given day is a product of the number of animals it admits and the length of time they stay in the shelter’s care (i.e. LOS).
Average Daily Population = Average Daily Admissions × Average Length of Stay
If two shelters take in the same number of animals each year, the shelter with the shorter average LOS will have fewer animals in care each day (Table 2.1).
Caring for fewer animals at a time allows shelters to provide better welfare and creates the capacity to provide care for animals who require longer stays.1 Or, when it is within the shelter’s capacity and mission to do so, shortening average LOS can allow the shelter to take in more animals or expand other services.
2.3.4 Pathway planning
LOS can be minimized through effective pathway planning. Pathway planning is a proactive process that anticipates the services and care an animal will require to achieve an appropriate outcome.12 A pathway is selected in consideration of available housing, personnel, resources, and the likelihood of achieving the outcome while maintaining good welfare. Planning ahead prevents needless delays that add days to a shelter stay.
Policies that detail which medical and behavioral conditions a shelter can treat help personnel make swift, measured decisions when an animal’s needs may be beyond their ability to provide care. Although legal holding periods and time in medical or foster care may extend the time in care, efficient planning of services can also decrease LOS for these animals.
For shelters with both an on-site and foster population, determining whether to pursue foster placement for an animal is a key part of pathway decision-making. Medical or behavioral care that can reasonably occur outside of the shelter, either in foster care or after adoption, should be identified to minimize time in the shelter environment. Regardless of whether animals are on site or in foster care, decision-making and animal movement must optimize LOS.
2.3.5 Population rounds
To ensure that each animal has a clear plan and that all needs and critical points of service are promptly met, the entire shelter population, including animals housed in foster or off-site, must be regularly assessed by knowledgeable personnel with decision-making ability and authority. The personnel involved in this assessment, often called population or ‘daily’ rounds, will vary based on the shelter population and organizational structure. Population rounds work best when participants include a small group of people who represent relevant departments or teams, including intake, medical, behavior, management, daily care, and outcome personnel (individuals may represent multiple areas). Participants collectively provide and consider all aspects of each animal’s pathway, needs, and next steps.
The population rounds team answers the following for each animal:
- How are you doing?
- What is your pathway?
■ Are there updates or concerns that change this pathway?
- What are your next steps?
The outcome of population rounds is a task list for each participant or team. Any needs identified during population rounds that could compromise welfare or extend the shelter stay must be addressed promptly. Although population rounds are recommended daily for most shelters, it is more important that population rounds occur frequently enough that animal care, including for those in foster, is not delayed.
Additionally, all animals physically in the shelter must be monitored daily to identify housing, care, or service needs. Monitoring these needs helps a shelter determine whether they are within or over their capacity for care. A shelter animal inventory, including all animals in foster care, should be taken and reconciled daily. This ensures that no animals are missing, data collection is accurate, and population levels are within capacity for care. This inventory can be taken during population rounds or daily monitoring.1
2.4 Monitoring population data
Keeping track of shelter metrics and population statistics over time is a key component of successful population management. Population level statistics are available as reports from shelter software programs or can be generated manually using commonly available spreadsheet programs. At a minimum, shelters must track monthly intake and outcome type for each species by age group.19
Data collection should include information about health and behavior status at intake and outcome. Tracking this information allows shelters to understand the effects of shelter care on animal health and well-being. For example, discovering a trend where animals that are healthy at the time of intake subsequently become ill warrants investigation into the shelter’s population management practices.20
LOS data, broken down by age category, species, status, and location, should be regularly analyzed to identify bottlenecks, mismatched resources, and capacity for care concerns.1,9 Population level data should be reviewed and analyzed regularly to ensure that operations align with the organization’s goals, purpose, and policies.9 For example, when an organization’s mandate is to admit stray, injured, or at-risk animals, redirecting healthy community cats to return-to-field services creates capacity to care for the animals that the organization is required to serve.21
Because local capacity to support animal welfare is maximized when organizations collaborate, population level metrics are ideally monitored as a community through transparent sharing of data. Sharing data can help communities strategically leverage resources, increase efficiency, and maximize impact for community animals and people. Organizations can share their data directly or participate in national data sharing databases such as Shelter Animals Count.22 Although useful for tracking shelter goals year over year, outcome-based metrics do not account for quality of life or animals still in the shelter’s care. Live release rates or save rates must be evaluated in the context of animal welfare and cannot be used alone as a measure of success.9 Aversion to euthanasia is not an excuse for crowding and poor welfare.
References
| 1. | Newbury S, Hurley K. Population Management. In: Miller L, Zawistowski S, eds. Shelter Medicine for Veterinarians and Staff. 2nd ed. Ames, IA: Wiley Blackwell; 2013:93–113. |
| 2. | Rewa OG, Stelfox HT, Ingolfsson A, et al. Indicators of Intensive Care Unit Capacity Strain: A Systematic Review. Crit Care. 2018;22(1):86. doi: 10.1186/s13054-018-1975-3 |
| 3. | Alalmai A, Arun A, Alalmai AA, Gunaseelan D. Operational Need and Importance of Capacity Management into Hotel Industry – A Review. Int J Adv Sci Technol. 2020;29(7):122–130. Accessed Dec 13, 2022. https://www.researchgate.net/publication/350616399. |
| 4. | Dybdall K, Strasser R, Katz T, et al. All Together Now: Group Housing for Cats. Appl Anim Behav Sci. 2003;11(1):816–825. doi: 10.1016/j.jfms.2009.03.001 |
| 5. | Hurley KF, Kraus S, Sykes JE. 17: Prevention and Managment of Infection in Canine Populations. In: Sykes JE, ed. Greene’s Infectious Diseases of the Dog and Cat. 5th ed. Amsterdam: Elsevier; 2022:197–203. |
| 6. | Karsten CL, Wagner DC, Kass PH, Hurley KF. An Observational Study of the Relationship between Capacity for Care as an Animal Shelter Management Model and Cat Health, Adoption and Death in Three Animal Shelters. Vet J. 2017;227:15–22. doi: 10.1016/j.tvjl.2017.08.003 |
| 7. | Janke N, Berke O, Flockhart T, Bateman S, Coe JB. Risk Factors Affecting Length of Stay of Cats in an Animal Shelter : A Case Study at the Guelph Humane Society, 2011–2016. Prev Vet Med. 2017;148(October):44–48. doi: 10.1016/j.prevetmed.2017.10.007 |
| 8. | National Animal Care and Control Association. Determining Kennel Staffing Needs. 2020. Accessed Dec 13, 2022. https://www.nacanet.org/determining-kennel-staffing-needs. |
| 9. | Scarlett JM, Greenberg MJ, Hoshizaki T. Every Nose Counts: Using Metrics in Animal Shelters. 1st ed. CreateSpace Independent Publishing Platform; 2017. Ithaca NY. |
| 10. | Ly LH, Gordon E, Protopopova A. Inequitable Flow of Animals In and Out of Shelters: Comparison of Community-Level Vulnerability for Owner-Surrendered and Subsequently Adopted Animals. Front Vet Sci. 2021;8:784389. doi: 10.3389/fvets.2021.784389 |
| 11. | Hobson SJ, Bateman S, Coe JB, Oblak M, Veit L. The Impact of Deferred Intake as Part of Capacity for Care (C4C) on Shelter Cat Outcomes. J Appl Anim Welf Sci. 2021;00(00):1–12. doi: 10.1080/10888705.2021.1894148 |
| 12. | Hurley K, Miller L. In: Miller L, Janeczko S, Hurley K, eds. Infectious Disease Management in Animal Shelters. 2nd ed. Hoboken, Chapter 1 Introduction to Infectious Disease Management in Animal Shelters 1–12, NJ: Wiley Blackwell; 2021. |
| 13. | Hurley KF. The Evolving Role of Triage and Appointment-Based Admission to Improve Service, Care and Outcomes in Animal Shelters. Front Vet Sci. 2022;9:809340. doi: 10.3389/fvets.2022.809340 |
| 14. | National Animal Control Association. Guideline on Appointment-Based Pet Intake into Shelters. Accessed Dec 13, 2022. https://www.nacanet.org/wp-content/uploads/2021/12/NACA-Guideline-on-Appointment-Based-Pet-Intake-into-Shelters.pdf. |
| 15. | Griffin B. Wellness. In: Miller L, Janeczko S, Hurley KF, eds. Infectious Disease Management in Animal Shelters. 2nd ed. Hoboken, NJ: Wiley Blackwell; 2021:13–45. |
| 16. | Lord LK, Wittum TE, Ferketich AK, Funk JA, Rajala-Schultz PJ. Search and Identification Methods that Owners Use to Find a Lost Dog. JAVMA. 2007;230(2):211–216. |
| 17. | Lord LK, Wittum TE, Ferketich AK, Funk JA, Rajala-Schultz PJ. Search and Identification Methods that Owners Use to Find a Lost Cat. JAVMA. 2007;230(2):217–220. |
| 18. | University of Wisconsin-Madison School of Veterinary Medicine Shelter Medicine Program. Support for Open Adoptions. Accessed Dec 13, 2022. https://www.uwsheltermedicine.com/library/resources/support-for-open-adoptions. |
| 19. | Shelter Animals Count. Basic Data Matrix. Accessed Dec 13, 2022. https://www.shelteranimalscount.org/wp-content/uploads/2022/02/BasicDataMatrix_SAC.pdf. |
| 20. | Scarlett J. Data Surveillance. In: Miller L, Janeczko S, Hurley K, eds. Infectious Disease Management in Animal Shelters. 2nd ed. Hoboken, NJ: Wiley Blackwell; 2021:46–58. |
| 21. | National Animal Care & Control Association. Animal Control Intake of Free-Roaming Cats. Accessed Dec 13, 2022. https://www.nacanet.org/wp-content/uploads/2021/03/Animal-Control-Intake-of-Free-Roaming-Cats.pdf. |
| 22. | Shelter Animals Count. Shelter Animals Count: Home. Accessed Dec 13, 2022. https://www.shelteranimalscount.org/ |
3. Animal Handling
3.1 General
Safe and humane handling is an essential part of supporting animal well-being. When fear and stress are minimized, animals are calmer and more willing to interact, resulting in safer and more successful interactions. Handling must be humane and appropriate for the individual animal and situation. Humane handling requires
- on-going observation and assessment of behavior with adjustments to the animal’s handling plan as needed
- appropriate choice and management of environment
- sufficient number of trained personnel
- suitable equipment readily available and in good working condition
Considering how animals perceive their environment and making adjustments to minimize potential stressors can reduce or prevent negative emotional responses. These adjustments might include a slow introduction, providing a hiding option during handling (e.g. with a towel), covering a table surface to improve traction, keeping voices low, and the use of gentle but consistent touch to reduce unpredictability.1,2 To create a positive emotional response to human handling, shelter personnel should offer high-value treats or food when handling animals or performing procedures. Treats and toys can engage, distract, and reward animals before, during, and immediately after handling.3,4 When needed, medication should be used to minimize fear, anxiety, and stress and enhance safety during handling5–9 (see Behavior).
3.2 Restraint
Resistance to handling is almost always the result of fear or anxiety. Improper or forceful use of restraint techniques and equipment can escalate a high stress situation, increasing the likelihood of animal or human injury.10 Gentle handling with minimal restraint can improve safety and compliance during care tasks for most animals. The minimal amount of physical restraint needed to accomplish necessary animal care without injury to people or animals must be used.11,12
Forceful restraint methods must not be used, except in extraordinary circumstances. Extraordinary circumstances include situations in which a human or animal is in immediate danger, and other low-stress handling options, sedation, or delays are not possible. Forceful restraint methods include scruffing cats12 or pinning dogs to the ground. For example, a short period of forceful restraint may be required for an animal that needs to be captured and removed from an unsafe environment. Techniques that rely on dominance theory, such as alpha rolls, are inhumane.5,11,13
Alternatives to forceful restraint include distraction with food or toys, positive reinforcement, use of towels, blocking visual stimuli, sedation, and proper use of humane handling equipment (Table 3.1). Selecting a quiet environment, preparing all necessary materials in advance, and involving a person the animal has a bond with can help minimize fear, anxiety, and stress and reduce the restraint required.14,15 If repeated handling is required, training the animal to allow common tasks or to cooperate with handling equipment such as the use of a muzzle is a valuable strategy. Use of sedatives or behavior medications can be the most humane and effective option for frightened, fractious, or feral animals for the delivery of necessary care.1
Handling must minimize the risk of escape. Attention to security of enclosures and carriers, building and vehicle exit points, and minimizing fearful stimuli that trigger flight behavior are important during daily care and when moving animals inside and outside the facility. Being recaptured after escape is profoundly stressful for many animals and creates additional risk of injury to the animal and personnel.4 Delaying handling to allow the animal to calm down can minimize stress and reduce the risk of escape.
3.3 Handling equipment
Using humane handling equipment minimizes animal stress during necessary procedures and daily care, prevents escape, and promotes animal and human safety. For example, rather than carrying a cat in their arms, personnel can transport cats through the shelter in carriers. A variety of humane equipment that facilitates animal handling with minimal or no hands-on contact must be available (Table 3.1). Handling equipment also has the potential to increase fear or injury if used in a forceful manner or not maintained in good working order.
Control poles (i.e. catch poles or rabies poles) are designed to keep a dog’s head at a safe distance from a handler. They are not meant to lift, push, or pull a dog and are not appropriate for routine use. Control poles must only be used when alternatives for handling dogs are insufficient to protect human safety. To prevent the need for daily removal of dogs that are not deemed safe to walk on a leash, double compartment housing is recommended.
Because control poles can cause significant injury and even death, it is unacceptable to use control poles on cats or small dogs. Any restraint method, including control poles, cat tongs, or slip-leads, that causes significant compression of the neck or thorax can cause substantial or life-threatening injury and profound emotional trauma in cats.4,12,16
Animals for whom handling equipment is necessary for long-term safe handling should receive positive reinforcement training to minimize fear, anxiety, and distress during its use.11
Aggressive behavior between dogs can occur unexpectedly for a variety of reasons, and humans can be severely injured when trying to intervene. Animal shelters must have written protocols and readily accessible equipment for breaking up dog fights to prevent human and animal injury. Equipment may include air horns, whistles, citronella spray, blankets, break sticks, panels, and water hoses17,18 (see Behavior).
3.4 Handling feral cats
Specific handling procedures are necessary for feral cats, including the use of live traps, cat dens, squeeze cages, trap dividers, purposely designed cage nets, and multi-compartment enclosures.16,19–21 This equipment permits personnel to safely sedate or anesthetize extremely fearful cats with injectable medication, to provide food and sanitation, to transfer cats from one enclosure to another, and to release outside, all without hands-on handling.
References
| 1. | Moffat K. Addressing Canine and Feline Aggression in the Veterinary Clinic. Vet Clin North Am – Small Anim Pract. 2008;38(5):983–1003. doi: 10.1016/j.cvsm.2008.04.007 |
| 2. | Griffin B. Fear Free Shelters. 2022. https://fearfreeshelters.com/. |
| 3. | Herron ME, Shreyer T. The Pet-Friendly Veterinary Practice: A Guide for Practitioners. Vet Clin North Am – Small Anim Pract. 2014;44(3):451–481. doi: 10.1016/j.cvsm.2014.01.010 |
| 4. | Janeczko S. Feline Intake and Assessment. In: Weiss E, Mohan-Gibbons H, Zawistowski S, eds. Animal Behavior for Shelter Veterinarians and Staff. Ames, IA: Elsevier Saunders; 2015:191–217. |
| 5. | Hammerle M, Horst C, Levine E, et al. 2015 AAHA Canine and Feline Behavior Management Guidelines. J Am Anim Hosp Assoc. 2015;51(4):205–221. doi: 10.5326/JAAHA-MS-6527 |
| 6. | Stevens BJ, Frantz EM, Orlando JM, et al. Efficacy of a Single Dose of Trazodone Hydrochloride Given to Cats Prior to Veterinary Visits to Reduce Signs of Transport- and Examination-Related Anxiety. J Am Vet Med Assoc. 2016;249(2):202–207. doi: 10.2460/javma.249.2.202 |
| 7. | van Haaften KA, Eichstadt Forsythe LR, Stelow EA, et al. Effects of a Single Preappointment Dose of Gabapentin on Signs of Stress in Cats during Transportation and Veterinary Examination. J Am Vet Med Assoc. 2017;251(10):1175–1181. doi: 10.2460/javma.251.10.1175 |
| 8. | Pankratz KE, Ferris KK, Griffith EH, Sherman BL. Use of Single-Dose Oral Gabapentin to Attenuate Fear Responses in Cage-Trap Confined Community Cats: A Double-Blind, Placebo-Controlled Field Trial. J Feline Med Surg. 2018;20(6):535–543. doi: 10.1177/1098612X17719399 |
| 9. | Erickson A, Harbin K, Macpherson J, Rundle K, Overall KL. A Review of Pre-Appointment Medications to Reduce Fear and Anxiety in Dogs and Cats at Veterinary Visits. Can Vet J. 2021;62(09):952–960. |
| 10. | Herron ME, Shofer FS, Reisner IR. Survey of the Use and Outcome of Confrontational and Non-Confrontational Training Methods in Client-Owned Dogs Showing Undesired Behaviors. Appl Anim Behav Sci. 2009;117(1–2):47–54. doi: 10.1016/j.applanim.2008.12.011 |
| 11. | Yin S. Low Stress Handling, Restraint and Behavior Modification of Dogs and Cats. Cattledog Publishing; 2009. Davis CA. |
| 12. | Rodan I, Dowgray N, Carney HC, et al. 2022 AAFP / ISFM Cat Friendly Veterinary Interaction Guidelines: Approach and Handling Techniques. J Feline Med Surg. 2022;24(11):1093–1132. |
| 13. | American Veterinary Society on Animal Behavior. Position Statement on the Use of Dominance Theory. 2008:1–4. Accessed Dec 13, 2022. https://avsab.ftlbcdn.net/wp-content/uploads/2019/01/Dominance_Position_Statement-download.pdf. |
| 14. | American Veterinary Society of Animal Behavior. Position Statement on Positive Veterinary Care: What Is a Positive Veterinary Experience? 2016. Accessed Dec 13, 2022. https://avsab.org/wp-content/uploads/2018/03/Positive-Veterinary-Care-Position-Statement-download.pdf. |
| 15. | Taylor S, Denis KS, Collins S, et al. 2022 ISFM/AAFP Cat Friendly Veterinary Environment Guidelines. J Feline Med Surger. 2022;24(11):1133–1163. |
| 16. | Levy JK, Wilford CL. Management of Stray and Feral Community Cats. In: Miller L, Zawistowski SL, eds. Shelter Medicine for Veterinarians and Staff. 2nd ed. Ames, IA; 2013:669–688. |
| 17. | Mullinax L, Sie K, Velez M. Inter-Dog Playgroup Guidelines. Shelter Playgroup Alliance.2019:4–65. |
| 18. | Association of Shelter Veterinarians. Position Statement: Playgroups for Shelter Dogs. 2019. Accessed Dec 13, 2022. https://avsab.org/wp-content/uploads/2018/03/Punishment_Position_Statement-download_-_10-6-. |
| 19. | Slater M. Behavioral ecology of free-roaming/community cats. In: Weiss E, Mohan-Gibbons H, Zawistowski S, eds. Animal Behavior for Shelter Veterinarians and Staff. 1st ed. Ames, IA: Wiley Blacklwell; 2015:102–128. |
| 20. | Griffin B. Care and Control of Community Cats. In: Little S, ed. The Cat: Clinical Medicine and Management. 1st ed. St. Louis, MO: Elsevier Saunders; 2011:1290–1309. John Wiley and Sons, Hoboken NJ. |
| 21. | Griffin B. Care and Control of Community Cats. In: Little S, ed. The Cat. 2011. |
4. Facilities
4.1 General
The facility plays a critical role in the care provided to animals who are admitted into animal shelters. While community-centered sheltering practices and foster programs are reducing the demand for in-shelter care in some areas, providing housing for animals remains an essential part of sheltering operations. Thoughtful planning and use of the shelter building and grounds are important parts of supporting the physical and emotional health of shelter populations while meeting the organization’s mission and goals.1 The shelter facility must include sufficient space to allow for the execution of essential shelter operations and programs as required by mission or mandate.
The quality and set-up of animal housing impacts every aspect of their experience within the facility and plays a pivotal role in managing disease.2 Poor housing is one of the greatest shortcomings observed in shelters and has a substantially negative impact on both health and well-being. Both the quantity and design of housing must be appropriate for the species, the number of animals receiving care, and the expected length of stay. Facility design and use must provide for proper separation of animals by species, predator/prey status, health status, and behavior. Housing in foster care should meet or exceed the guidelines for in-shelter housing.
4.2 Primary enclosures
A primary enclosure is an area of confinement such as a cage, kennel, or housing unit where an animal spends the majority of their time. Shelters must have a variety of housing units available to meet the individual needs of animals, including physical, behavioral, and medical needs. These needs will vary based on species, life stage, individual animal personality, prior socialization, and past experience.1 Appropriate primary enclosures provide complexity and allow choice within the environment to help support positive welfare3 (see Behavior).
The primary enclosure must be structurally sound and maintained in safe, working condition to prevent injury and escape. There can be no sharp edges, gaps, or other defects that could cause injury or trap a limb or other body part. Primary enclosures with wire-mesh bottoms or slatted floors are unacceptable because they can cause pain, discomfort, and injury. Enclosure sides that are entirely wire or chain-link increase the risk of disease transmission, animal stress, and injury. Solid barriers are recommended where animal contact can occur.
The use of cages or crates intended for short-term, temporary confinement or travel is also unacceptable as primary enclosures. These include airline crates, transport carriers, live traps, and wire crates. It is unacceptable to stack or arrange enclosures in a manner that increases animal stress and discomfort, compromises ventilation, or allows for waste material contamination between housing units.
4.2.1 Individual primary enclosure size
Animals must be able to make normal postural adjustments within their primary enclosure, including standing and walking several steps, sitting normally, laying down at full body length, and holding the tail completely erect.1,3–6 Primary enclosure size significantly impacts overall health and well-being. Larger enclosures generally provide animals more choice, permit additional enrichment, and make it possible to safely interact with people and other animals for socialization or cohousing. In cats, sufficiently sized housing reduces stress and respiratory disease incidence.7,8 Individual adult cat housing that is less than 8 ft2 (0.75 m2) of floor space is unacceptable.8 Ideally, individual cat housing provides 11 ft2 (1.0 m2) or more of floor space.7 For dogs, the minimum recommended kennel dimensions differ widely based on body size.9
The primary enclosure must allow animals to sit, sleep, and eat away from areas of their enclosures where they defecate and urinate.8 Housing with two or more appropriately sized compartments provides this separation and gives animals more choice and control over their environment and interactions. It also facilitates spot cleaning, reduces fomite transmission, and increases personnel safety3,5 (see Sanitation). Because of all these benefits, multi-compartment enclosures should be provided for the majority of animals housed in the shelter.
Multi-compartment housing is particularly important for newly admitted, fractious, quarantined, sick, and juvenile animals. Enriched room-sized primary enclosures (i.e. real-life rooms) may also benefit from separate elimination areas. Single compartment housing may be necessary for animals with specific medical conditions, which increases the importance of enhanced in-kennel enrichment and supervised out of kennel time (see Behavior).
Cats prefer spending time on raised surfaces and high structures rather than being on the floor.10,11 Cat housing units should be elevated off the floor. Housing cats at human eye level reduces stress, facilitates positive interactions with personnel and visitors, and improves ease of monitoring.5,6,12 Cat cages should face away from each other or be spaced more than 4 ft (1.2 m) apart to prevent droplet transmission of respiratory pathogens while sneezing, coughing, or vocalizing.13–15
Primary enclosures with indoor–outdoor access are ideal for most animals, especially when held long term. Some shelters in temperate climates may have primary enclosures that are fully outdoors. Enclosures that include outdoor space must protect animals from adverse weather; provide choice for thermoregulation; protect from predators; and prevent escape, theft, or harassment. It is recommended that all enclosed outdoor spaces have double-door entry points to keep animals safe and reduce the risk of escape.
4.2.2 Primary enclosure set-up
In addition to the size and structural layout, the set-up of the enclosure and care items provided are important in meeting the welfare needs of shelter animals (Figures 4.1 & 4.2).1 The enclosure needs to be large enough to accommodate the necessary set-up without impeding the animal’s ability to move or stretch.
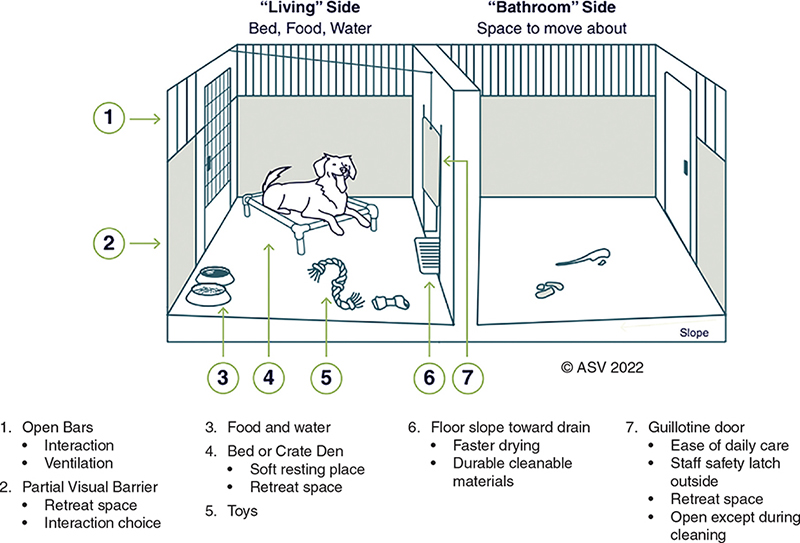
Figure 4.1. Canine primary enclosure set-up
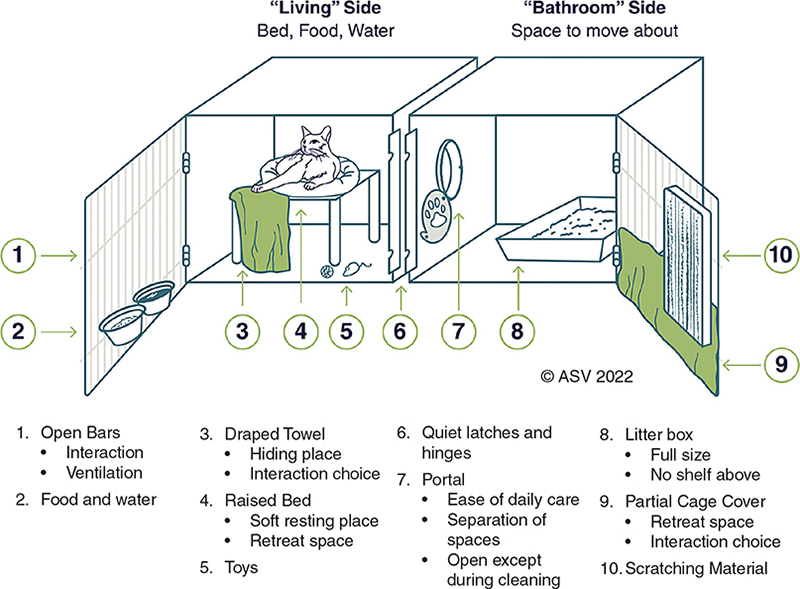
Figure 4.2. Feline primary enclosure set-up
All dogs should be given the opportunity to hide within their enclosure, especially young, small, fearful, and anxious animals. Options for canine hiding areas include a covered crate within the enclosure or a visual barrier over part of the kennel front.
A soft resting place that elevates animals off of the floor should be made available for all animals to ensure comfort, keep animals dry, and support thermoregulation.
All cats must be given the opportunity to hide within their enclosure. A hiding place provides the choice to be seen or not seen and a place to feel safe and protected.11,16 Options for feline hiding places include feral cat dens, perches covered with towels, cardboard boxes, and partial coverings over enclosure doors. Cats with hiding places spend less time trying to hide and are more likely to approach adopters.17,18
To ensure that cats can display natural behaviors, feline primary enclosures must allow scratching, climbing, and perching. Cats must have a litter box large enough to comfortably accommodate their entire body and allow for proper posturing.19,20 Litter boxes that are too small impact welfare and potentially lead to house soiling behavior.20
4.2.3 Additional considerations
Appropriately sized, enriched primary enclosures are critical for all animals regardless of their length of stay in the shelter. Housing that provides animals with additional space, enrichment, and choice within their enclosure must be provided for animals remaining in the shelter long-term (i.e. more than 2 weeks). Foster care, while beneficial for many animals, can be particularly valuable when animals require a longer length of stay, such as protracted legal holds or long-term medical care.
Animals for whom handling poses an acute welfare or safety risk need to be housed in enclosures that allow humane, touch-free daily care (i.e. multi-compartment). It is unacceptable to house animals in an enclosure that would require the use of forceful animal handling equipment for daily cleaning and care (see Animal Handling).
Except for a brief, emergency situation, it is unacceptable to house animals in facility spaces not intended for animal housing (e.g. bathrooms and hallways). Shelters may have multiuse spaces such as offices set up for animal housing; these planned spaces differ from unplanned practices such as placing temporary kennels in areas unequipped for sanitation or delivery of care.
Tethering is an unacceptable method of confinement for any animal.21 Tethering can cause significant stress and frustration and is best avoided even when used briefly during the cleaning of primary enclosures. Multi-compartment enclosures, thoughtful timing of walks and playgroups, or the use of securely enclosed exercise areas are good alternatives to tethering.
4.3 Cohousing
Cohousing, or keeping more than one animal in an enclosure, can improve animal welfare in some circumstances by facilitating social contact with other animals of the same species.22–29 However, cohousing also known as group housing, is not suitable for every situation. Mental and physical benefits of cohousing need to be carefully weighed against risks to health and safety. If shelters are cohousing animals, they need to prioritize animal well-being and keep population levels within their capacity for care.
4.3.1 Cohousing enclosure set-up
The size and set-up of enclosures used for cohousing require special considerations. The size of a primary enclosure for cohousing must allow each animal to express a variety of normal behaviors and maintain distance from roommates when they choose to do so. Meeting these needs often requires more space per animal than required for individual enclosures, particularly when unfamiliar animals are cohoused. The optimal space requirements for cohousing vary based on species, as well as size, activity level, and behavior.27 A minimum of 18 ft2 (1.7 m2) of floor space per adult cat should be provided for cohousing.4
Quality and complexity of cohousing environments is essential to support the welfare of all animals living in the enclosure.26,30,31 Appropriate resources (e.g. food, water, bedding, litter boxes, and toys) must be provided to minimize competition or resource guarding and ensure access by all cohoused animals. Functional space can be maximized by spacing resources out throughout the enclosure. For cohoused cats, a variety of elevated resting perches and hiding places must be provided to increase complexity and choice within the living space.22,32–36 The ability to choose resting places, social interactions, elimination spaces, and toys contributes to behavioral stability within groups.
Cohousing areas may require enhanced measures to prevent escape. Double door entry at the enclosure’s entrance can provide additional protection when entering or exiting. When housed in a retrofitted area, cats may be able to dislodge ceiling panels or duct covers unless care is taken to secure them.37
4.3.2 Selecting animals for cohousing
Random cohousing of animals in shelters is an unacceptable practice.25 Cohousing requires careful selection of animals by trained personnel to balance the benefits and risks for individual animals and the group. Unrelated or unfamiliar animals must not be cohoused until health and behavior are assessed.27
When cohoused, animals need to be intentionally matched for age, sex, health, and behavioral compatibility. Monitoring after introduction is essential to recognize signs of stress or negative interactions (e.g. guarding food or other resources) that may necessitate separation. Given their increased welfare needs, animals predicted to have longer lengths of stay may benefit most from cohousing, particularly when foster care is not available.
Regardless of the size of the enclosure, no more than six adult cats should be cohoused in a primary enclosure.5 When cohousing is indicated, pairs are preferred for dogs to maximize safety and biosecurity, and no more than two to four adult dogs should be cohoused in a primary enclosure.3 Larger groups of any species are challenging to monitor and increase the risk of conflict and infectious disease transmission. It is preferable to cohouse the minimum number of adult animals together needed to achieve a social benefit.
Housing young puppies and kittens with their mother and littermates is important for physical and emotional development, as well as the establishment of species-specific behaviors. Because of their susceptibility to infectious disease, puppies and kittens under 20 weeks of age must not be cohoused with unfamiliar animals except when the benefits outweigh the risks for all animals involved.38 For example, after a careful medical and behavioral assessment, a single orphaned kitten or puppy may be paired with another orphan or a surrogate mother (see Behavior).
Introducing new animals can result in stress for individuals and the group. Dogs should be introduced outside of their primary enclosures in pairs or groups to determine compatibility prior to cohousing.3,27 In addition, turnover within groups must be minimized to reduce stress and social conflicts as well as the risk of infectious disease exposure and transmission.22,39,40
The use of smaller enclosures with fewer animals, rather than large rooms with large groups of animals, minimizes the need for frequent introductions, group reorganization, and allows for more effective monitoring.41,42 Smaller cohousing spaces facilitate an ‘all-in/all-out’ approach, where all animals leave before more are added. This strategy allows enclosures to be completely sanitized before a new group of animals moves in and eliminates the risks associated with new introductions.
4.3.3 Monitoring cohoused animals
Individual animals and group dynamics must be monitored to recognize signs of stress and social conflicts in cohousing enclosures.24,43 Monitoring, especially after a new animal is introduced into a group and during feeding time, is critical to ensure that all animals are benefitting. In addition to daily monitoring for resource guarding and other signs of social conflict, regular physical examinations including measurement of body weight can ensure that cohoused animals are not suffering due to unrecognized social conflicts.
Not all animals are well suited to cohousing. Individual enriched housing must be provided for animals who are fearful or behave aggressively toward other animals, are stressed by the presence of other animals, require individual monitoring, or are ill and require treatment that cannot be provided in cohousing.22,41 Cohousing animals who fight with one another is unacceptable.
4.4 Isolation housing
Shelters must have a means of isolating infectious animals from the general population to prevent the spread of infectious disease. Isolation housing must meet the medical and behavioral needs of ill animals, including being of sufficient size with appropriate set-up. Different species must not be housed within the same isolation room.1
Separate isolation areas must be provided for animals with different highly contagious diseases to prevent coinfections with multiple pathogens. For example, dogs with parvovirus infection need to be separated from those with infectious respiratory disease. This separation is more readily accomplished in flexible-use rooms with a smaller number of enclosures. Animals that already have coinfections (e.g. ringworm and upper respiratory infection) will need veterinary input to determine the most appropriate isolation housing.
To avoid exposure of healthy animals to sick animals, isolation rooms must be designed so that they do not open directly into another animal housing area. A corridor or vestibule can be used to access isolation rooms and also serve as a space to put on and remove personal protective equipment (PPE). Isolation rooms should have access to a sink for handwashing and be set up with space for treatments, examinations, and storage for dedicated supplies.
Isolation rooms must be clearly labeled to indicate current use and necessary precautions. Human and animal traffic through isolation spaces should be limited1 (see Medical Health). Limiting foot traffic reduces the risk of spreading infection to others outside of isolation and reduces stress for ill animals during recovery. Ideally, isolation rooms are designed with windows to allow observation of animals from a corridor without needing to repeatedly enter the room.1
When no isolation options exist, makeshift separation can be accomplished by housing contagious dogs at least 25 ft (7.6 m) from unaffected dog enclosures and covering enclosure doors.44 Contagious ill cats may be separated from others in their individual enclosures in a general ward if they can be cared for without fomite transmission to other cats. These options will not be as effective at reducing transmission as isolation.
4.5 Surfaces and drainage
Primary enclosures and all animal areas must be able to be fully sanitized and withstand repeated cleanings. Nonporous surfaces are important in cages and kennels, as well as high traffic areas such as walkways or play rooms. A sealed, impermeable surface, such as resinous epoxy or resinous urethane, is recommended for shelter flooring and should be considered for new facilities. Linoleum or tiles may be acceptable flooring in low-risk areas. However, these materials are less durable, more challenging to sanitize due to seams and grout lines, and may harbor infectious pathogens in areas that are damaged or worn. Regardless of flooring type, points where walls meet floors should be sealed to prevent water intrusion and the accumulation of organic matter and pathogens.
Drainage systems must be designed to prevent standing water and cross-contamination of waste between housing units. Many design options exist. To aid in this effort, floors should be gently sloped to enable waste and water to run into the drains, particularly in animal housing areas. Drain covers must be designed to prevent injury or escape and should be easily removable for routine cleaning. Similarly, outdoor primary enclosures or portions of primary enclosures that are outdoors must have nonporous, durable floors that allow for sanitation and proper drainage.
4.6 Heating, ventilation, and air quality
It is essential that housing areas allow each animal to comfortably maintain normal body temperature.9,45 To ensure humane and comfortable conditions, environmental temperature must be maintained between 64°F (18°C) and 80°F (26.6°C.)38,45 Breed, body condition, medical health, haircoat, facial conformation, and age impact an animal’s ability to regulate their body temperature.
Animals must be monitored individually to ensure the environmental temperature is comfortable, and necessary measures must be taken if an animal appears too cold or too hot. If an animal cannot be kept comfortable with adjustments to the thermostat and airflow, additional measures need to be taken. These might include provision of additional bedding if too cold, providing frozen treats or ice if too hot, or relocating the animal. The relative humidity should be maintained between 30 and 70%.47–49
Proper ventilation removes heat, dampness, odor, airborne microbes, and pollutant gasses such as ammonia and carbon dioxide while allowing for the introduction of fresh, oxygenated air. Fresh air is essential for the well-being of shelter animals and personnel, as well as for limiting the spread of infectious disease.50 Ventilation must be maintained at a high enough rate to ensure adequate air quality in all areas of the shelter including in the primary enclosure. Ventilation rates may need to be adjusted seasonally, especially if air movement occurs primarily through active heating or cooling.
Ventilation must not compromise recommended ambient temperatures.38 The standard recommendation for ventilation of animal facilities is between 10 and 20 room air exchanges per hour with fresh air.38,51–53 Ventilation requirements vary depending on population density and presence of pollutants in the air. A facility may require a higher ventilation rate when it is at full capacity compared to when it is relatively empty, as animals themselves are a major source of heat, humidity, and carbon dioxide. All ventilation systems must be regularly maintained based on manufacturer recommendations. Carbon dioxide monitors may be useful in monitoring the success of ventilation equipment and use.
To improve ventilation, barred enclosure doors are recommended over plexiglass doors or fully enclosed units. When housing units are fully enclosed, they require individual-unit mechanical ventilation. Barred doors improve air flow and also allow for adopter interaction and behavior training.
Because canine respiratory pathogens can be easily transmitted through the air, air from isolation areas should be exhausted outside and not recirculated. Separate air exchanges for feline isolation areas are a lower priority since cats do not readily transmit pathogens through the air.14,15
Air purification technologies, such as ultraviolet germicidal irradiation (UVGI), may act as an adjunct to a traditional HVAC system to improve indoor air quality. However, ultraviolet irradiation must not be relied on as the sole method for ensuring good air quality or infectious disease prevention.54–62 Although attention to ventilation and air quality is important, it will not overcome the harmful effects of inadequate housing, poor sanitation, or lax population management.
4.7 Noise control
Noise must be minimized in animal housing areas. Cat and dog hearing is sensitive, and noise levels that are uncomfortable for humans are likely to be very uncomfortable for animals (see Behavior). Noise and vibration-producing equipment and mechanical systems should be located as far away from animal housing as possible.63
Even reasonable volumes may be stressful for shelter animals, particularly if sounds are sudden or unpredictable such as the slamming of cage doors or tossing of metal bowls.64,65 Prevention and mitigation strategies to minimize the impact of noise should be implemented in facility design, added to existing facilities, and incorporated into shelter operations. These strategies can include arrangement of cages; material selection for cages, doors, and latches; and decisions about where to house individual animals.
Barking can be a significant source of shelter noise. Appropriate facility design, environmental management, enrichment strategies, and behavior modification can dramatically reduce noise levels related to barking.66–68 Because the causes and solutions to barking are multifactorial, preventing visual contact between dogs should not be used as a sole strategy to reduce barking.69,70
4.8 Lighting
Lighting should promote a safe working environment and effective observation of animals and the enclosure. Facilities should be designed to offer as much natural light as possible. Exposure to sunlight in a manner that maintains daily circadian rhythms improves health and well-being for animals and for shelter personnel.71 When natural lighting is not available and artificial light is used, it should approximate natural light in duration and intensity to support circadian rhythms.72 If it is necessary to keep lights on after dark for safety or by regulation, a fixture that emits red-orange light is preferred. Because of the way dog and cat eyes function, a red light creates a darker space for animals at night, allowing them to sleep more normally.71
4.9 Enrichment spaces
Dedicated indoor or outdoor enrichment, exercise, and training spaces allow shelters to safely provide opportunities that improve welfare for animals. These spaces need to be clearly marked, prevent escape, provide protection from the elements, and limit exposure to disease and parasites. All enclosed outdoor spaces should have double door entry points to keep animals safe and reduce the risk of escape.
4.10 Intake spaces
Designed appropriately, shelter lobbies provide a welcoming environment for clients and help reduce animal stress. Shelter admission areas should be separated from adoptions and other client-facing areas.51 If a different space is not available, placing a divider within the lobby or scheduling intake appointments outside of adoption hours can functionally separate admissions from adoptions.
Animal well-being during the admission process is supported by creating separate species areas within the lobby and intake examination space.6,8,51,71 To allow for safe and efficient processes, animal intake should occur in a designated quiet space away from the main pattern of foot traffic.73 Cages and kennels in intake areas should only hold animals until their initial intake assessment has been completed.6,8 Intake rooms should have elevated surfaces to place animals in carriers off of floor level.8,10,74
4.11 Drop boxes
The use of ‘drop boxes’ where live animals are placed in unmonitored receptacles for later intake is unacceptable. This practice can result in safety risks for humans and animals, animal suffering, infectious disease exposure, or death. Alternatives for community animals requiring after hours emergency care include posting on-call phone numbers for animal services, creating drop-off arrangements with police departments, or creating care agreements with local veterinary emergency clinics.
4.12 Facility design and planning
Well-designed shelter facilities support the well-being of animals and personnel and allow smooth and efficient operations. In order to meet the changing needs of the community and services offered by the shelter, flexibility in operational and spatial use should be incorporated into designs for remodeling and new facilities. Areas that can be readily adapted for multiple purposes over time can reduce the need for future renovations. When designing a new facility or undertaking a significant renovation, shelters should consult with a shelter veterinarian and an architect experienced in shelter design.
Shelters must avoid large warehouse type rooms when designing housing. Instead, multiple smaller rooms with fewer primary enclosures per area are strongly preferred.75 Small wards reduce noise, limit disease exposure and transmission, provide flexibility in meeting individual animal needs, and permit close monitoring of individual animals.
When remodeling or planning a new facility, the movement of animals, people, and supplies should be incorporated into the design. For example, placing housing for difficult to handle dogs close to the facility entry point will improve personnel and animal safety. Animal shelter design should provide an environment that also serves the needs of personnel and clients. Areas for training, work breaks, meetings, and private discussions support personnel well-being, client–staff interactions, and client–animal interactions.
References
| 1. | Griffin B. Wellness. In: Miller L, Janeczko S, Hurley KF, eds. Infectious Disease Management in Animal Shelters. 2nd ed. Hoboken, NJ: Wiley Blackwell; 2021:13–45. |
| 2. | Hurley K, Miller L. In: Miller L, Janeczko S, Hurley K, eds. Chapter 1 Introduction to Infectious Disease Management in Animal Shelters. 2nd ed. Hoboken, NJ: Wiley Blackwell; 2021: 1–12. |
| 3. | Hubrecht R, Wickens S, Kirkwood J. The Welfare of Dogs in Human Care. In: Serpell J, ed. The Domestic Dog: Its Evolution, Behavior and Interactions with People. 2nd ed. Cambridge: Cambridge University Press; 2016:271–299. |
| 4. | Wagner D, Newbury S, Kass P, Hurley K. Elimination Behavior of Shelter Dogs Housed in Double Compartment Kennels. PLoS One. 2014;9(5):5–9. doi: 10.1371/journal.pone.0096254 |
| 5. | Wagner D, Hurley K, Stavisky J. Shelter Housing for Cats: Principles of Design for Health, Welfare And Rehoming. J Feline Med Surg. 2018;20(7):635–642. doi: 10.1177/1098612X18781388 |
| 6. | Wagner D, Hurley K, Stavisky J. Shelter Housing for Cats: 2. Practical Aspects of Design and Construction, and Adaptation of Existing Accommodation. J Feline Med Surg. 2018;20(7):643–652. doi: 10.1177/1098612X18781390 |
| 7. | Kessler MR, Turner DC. Effects of Density and Cage Size on Stress in Domestic Cats (Felis Silvestris Catus) Housed in Animal Shelters and Boarding Catteries. Anim Welf. 1999;8(3):259–267. |
| 8. | Wagner DC, Kass PH, Hurley KF. Cage Size, Movement In and Out of Housing During Daily Care, and Other Environmental and Population Health Risk Factors for Feline Upper Respiratory Disease in Nine North American Animal Shelters. PLoS One. 2018;13(1):1–15. doi: 10.1371/journal.pone.0190140 |
| 9. | New Zealand Ministry for Primary Industries: Regulation and Assurance Branch. Code of Welfare: Dogs. 2018:1–45. Accessed Dec 13, 2022. https://www.agriculture.govt.nz/dmsdocument/1445-pigs-animal-welfare-code-of-welfare. |
| 10. | McCobb EC, Patronek GJ, Marder A, Dinnage JD, Stone MS. Assessment of Stress Levels Among Cats in Four Animal Shelters. JAVMA. 2005;226(4):548–555. doi: 10.2460/javma.2005.226.548 |
| 11. | Stella J, Croney C. Coping Styles in the Domestic Cat (Felis Silvestris Catus) and Implications for Cat Welfare. Animals. 2019;9(6):1–20. doi: 10.3390/ani9060370 |
| 12. | Fantuzzi JM, Miller KA, Weiss E. Factors Relevant to Adoption of Cats in an Animal Shelter. J Appl Anim Welf Sci. 2010;13(2):174–179. doi: 10.1080/10888700903583467 |
| 13. | Povey RC, Johnson RH. Observations on the Epidemiology and Control of Viral Respiratory Disease in Cats. J Small Anim Pract. 1970;11(7):485–494. doi: 10.1111/j.1748-5827.1970.tb05599.x |
| 14. | Gaskell RM, Wardley RC. Feline Viral Respiratory Disease: A Review with Particular Reference to its Epizootiology and Control. J Small Anim Pract. 1977;19(1–12):1–16. doi: 10.1111/j.1748-5827.1978.tb05452.x |
| 15. | Wardley RC, Povey RC. Aerosol Transmission of Feline Calciciviruses. An Assessment of Its Epidemiological Importance. Br Vet J. 1977;133(5):504–508. doi: 10.1016/S0007-1935(17)33993-3 |
| 16. | Ellis JJ, Stryhn H, Spears J, Cockram MS. Environmental Enrichment Choices of Shelter Cats. Behav Processes. 2017;141(April):291–296. doi: 10.1016/j.beproc.2017.03.023 |
| 17. | Stella JL, Croney CC, Buffington CT. Behavior and Welfare of Domestic Cats Housed in Cages Larger than U.S. Norm. J Appl Anim Welf Sci. 2017;20(3):296–312. doi: 10.1080/10888705.2017.1317252 |
| 18. | Kry K, Casey R. The Effect of Hiding Enrichment on Stress Levels and Behaviour of Domestic Cats (Felis Sylvestris Catus) in a Shelter Setting and the Implications for Adoption Potential. Anim Welf. 2007;16:375–383. |
| 19. | Carney HC, Sadek TP, Curtis TM, et al. AAFP and ISFM Guidelines for Diagnosing and Solving House-Soiling Behavior in Cats. J Feline Med Surg. 2014;16(7):579–598. doi: 10.1177/1098612X14539092 |
| 20. | Guy NC, Hopson M, Vanderstichel R. Litterbox Size Preference in Domestic Cats (Felis Catus). J Vet Behav Clin Appl Res. 2014;9(2):78–82. doi: 10.1016/j.jveb.2013.11.001 |
| 21. | Humane Society of the United States. Chaining and Tethering Dogs FAQ. Accessed Dec 13, 2022. Accessed Dec 13, 2022. https://www.humanesociety.org/resources/chaining-and-tethering-dogs-faq. |
| 22. | Griffin B, Hume K. Recognition and Management of Stress in Housed Cats. In: August J, ed. Consultations in Feline Internal Medicine. 5th ed. Philadelphia, PA: Elsevier Saunders; 2006:717–734. |
| 23. | Kessler MR, Turner DC. Stress and Adaptation of Cats (Felis Silvestris Catus) Housed Singly, In Pairs and In Groups in Boarding Catteries. Anim Welf. 1997;6(3):243–254. |
| 24. | Mertens PAP, Unshelm J. Effects of Group and Individual Housing on the Behavior of Kennled Dogs in Animal Shelters. Anthrozoos. 1996;9(1):40–51. doi: 10.2752/089279396787001662 |
| 25. | Wells DL. A Review of Environmental Enrichment for Kennelled Dogs, Canis Familiaris. Appl Anim Behav Sci. 2004;85(3–4):307–317. doi: 10.1016/j.applanim.2003.11.005 |
| 26. | Hubrecht RC, Serpell JA, Poole TB. Correlates of Pen Size and Housing Conditions on the Behaviour of Kennelled Dogs. Appl Anim Behav Sci. 1992;34(4):365–383. doi: 10.1016/S0168-1591(05)80096-6 |
| 27. | Grigg EK, Marie Nibblett B, Robinson JQ, Smits JE. Evaluating Pair Versus Solitary Housing in Kennelled Domestic Dogs (Canis Familiaris) Using Behaviour and Hair Cortisol: A Pilot Study. Vet Rec Open. 2017;4(1):1–14. doi: 10.1136/vetreco-2016-000193 |
| 28. | McMillan FD. The Psychobiology of Social Pain: Evidence for a Neurocognitive Overlap with Physical Pain and Welfare Implications for Social Animals with Special Attention to the Domestic Dog (Canis Familiaris). Physiol Behav. 2016;167:154–171. doi: 10.1016/j.physbeh.2016.09.013 |
| 29. | Hennessy MB, Willen RM, Schiml PA. Psychological Stress, Its Reduction, and Long-Term Consequences: What Studies with Laboratory Animals Might Teach Us about Life in the Dog Shelter. Animals. 2020;10:2061. doi: 10.3390/ani10112061 |
| 30. | Griffin B. DNU: Feline Care in the Animal Shelter. In: Shelter Medicine for Veterinarians and Staff. 2nd ed. Oxford; 2013:145–184. doi: 10.1002/9781119421511.ch10 |
| 31. | Rochlitz I. Recommendations for the Housing of Cats in the Home, in Catteries and Animal Shelters, in Laboratories and in Veterinary Surgeries. J Feline Med Surg. 1999;1(3):181–191. doi: 10.1016/S1098-612X(99)90207-3 |
| 32. | Dowling JM. All Together Now: Group Housing for Cats. Anim Shelter. 2003:13. |
| 33. | Overall K. Recognizing and Managing Problem Behavior in Breeding Catteries. In: Consultations in Feline Internal Medicine. 1997:3. |
| 34. | Rochlitz I, Podberscek A, Broom D. Welfare of Cats in a Quarantine Cattery. Vet Rec. 1998;143:35–39. doi: 10.1017/CBO9781107415324.004 |
| 35. | de Oliveira A, Tercariol C, Genaro G. The Use of Refuges by Communally Housed Cats. Animals. 2015;5(2):245–258. doi: 10.3390/ani5020245 |
| 36. | Desforges EJ, Moesta A, Farnworth MJ. Effect of a Shelf-Furnished Screen on Space Utilisation and Social Behaviour of Indoor Group-Housed Cats (Felis Silvestris Catus). Appl Anim Behav Sci. 2016;178:60–68. doi: 10.1016/j.applanim.2016.03.006 |
| 37. | Griffin B. Population Wellness: Keeping Cats Physically and Behaviorally Healthy. In: Little S, ed. The Cat: Clinical Medicine and Management. 1st ed. St. Louis, MO: Elsevier Saunders; 2012:1312–1356. |
| 38. | Van Sluyters RC, Ballinger Mi, Bayne K, Al E. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington, DC: Institute for Laboratory Animal Research (ILAR); 2003. |
| 39. | Crowell-Davis SL, Curtis TM, Knowles RJ. Social Organization in the Cat: A Modern Understanding. J Feline Med Surg. 2004;6(1):19–28. doi: 10.1016/j.jfms.2003.09.013 |
| 40. | Finka LR, Ellis SLH, Stavisky J. A Critically Appraised Topic (CAT) to Compare the Effects of Single and Multi-Cat Housing on Physiological and Behavioural Measures of Stress in Domestic Cats in Confined Environments. BMC Vet Res. 2014;10:73. doi: 10.1186/1746-6148-10-73 |
| 41. | Kessler MR, Turner DC. Socialization and Stress in Cats (Felis Silvestris Catus) Housed Singly and in Groups in Animal Shelters. Anim Welf. 1999;8(1):15–26. |
| 42. | The Welfare of Cats (AWNS 3). Rochlitz I, ed. Dordrecht, Netherlands: Springer; 2007. doi: 10.1201/b21911 |
| 43. | Arhant. Assessment of Behavior and Physical Condition of Shelter Cats as Animal-Based Indicators of Welfare. J Vet Behav. 2015;10(5):399–406. doi: 10.1016/j.jveb.2015.03.006 |
| 44. | Sykes JE. Canine Viral Respiratory Infections Etiology and Epidemiology. In: Sykes JE, ed. Canine and Feline Infectious Diseases. First. St Louis, MO: Elsevier; 2014:170–181. |
| 45. | American Veterinary Medical Association. AVMA Policy: Companion Animal Care Guidelines. Accessed Dec 13, 2022. https://www.avma.org/policies/companion-animal-care-guidelines. |
| 46. | National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (U.S.). Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press; 2011. |
| 47. | United States Department of Agriculture Animal and Plant Health Inspection Service. USDA Animal Care: Animal Welfare Act and Animal Welfare Regulations ‘Blue Book’. 2019:205. Accessed Dec 13, 2022. https://market.android.com/details?id=book-0zUzmJ32rvQC%0A https://books.google.com/books/about/USDA_Animal_Care_Animal_Welfare_Act_and.html?hl=&id=zgC6ybZ0RKsC. |
| 48. | Arundel AV, Sterling EM, Biggin JH, Sterling TD. Indirect Health Effects of Relative Humidity in Indoor Environments. Environ Health Perspect. 1986;65(3):351–361. doi: 10.1289/ehp.8665351 |
| 49. | Ahlawat A, Wiedensohler A, Mishra SK. An Overview on the Role of Relative Humidity in Airborne Transmission of Sars-Cov-2 in Indoor Environments. Aerosol Air Qual Res. 2020;20(9):1856–1861. doi: 10.4209/aaqr.2020.06.0302 |
| 50. | Cat Fanciers Association. CFA Cattery Standard Minimum Requirements. 2019. Accessed Dec 13, 2022. http://cfa.org/breeders/catteries/catterystandards.aspx. |
| 51. | Schlaffer L, Bonacci P. Shelter Design. In: Miller L, Zawistowski S, eds. Shelter Medicine for Veterinarians and Staff. 2nd ed. Ames, IA: Wiley Blackwell; 2013:21–35. |
| 52. | Council of Europe. European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes. 2009. Accessed Dec 13, 2022. http://www.coe.int/en/web/conventions/full-list/-/conventions/treaty/123. |
| 53. | Johnson T. The Animal shelter building: design and maintenance of a healthy and efficient facility. In: Miller L, Zawistowski SL, eds. Shelter Medicine for Veterinarians and Staff. First. Hoboken, NJ: Blackwell; 2004:55–66. |
| 54. | Pearce-Walker JI, Troup JI, Ives R, et al. Investigation of the Effects of an Ultraviolet Germicidal Irradiation System on Concentrations of Aerosolized Surrogates for Common Veterinary Pathogen. Am J Vet Res. 2020;81(6):506–513. doi: 10.2460/ajvr.81.6.506 |
| 55. | Tomb RM, Maclean M, Coia JE, et al. New Proof-of-Concept in Viral Inactivation: Virucidal Efficacy of 405 nm Light Against Feline Calicivirus as a Model for Norovirus Decontamination. Food Environ Virol. 2017;9(2):159–167. doi: 10.1007/s12560-016-9275-z |
| 56. | Nuanualsuwan S, Mariam T, Himathongkham S, Cliver DO. Ultraviolet Inactivation of Feline Calicivirus, Human Enteric Viruses and Coliphages. Photochem Photobiol. 2002;76(4):406–410. doi: 10.1562/0031-8655(2002)076<0406:uiofch>2.0.co;2 |
| 57. | Rutala WA, Weber DJ. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008: update May 2019. Centers for Disease Control and Prevention: Department of Health and Human Services 2020:8–163. |
| 58. | Kim D, Kang D. UVC Led Irradiation Effectively Inactivates Aerosolized Viruses. Appl Environ Microbiol. 2018;84(17):1–11. doi: 10.1016/B978-1-4377-0795-3.00017-X |
| 59. | Thurston-Enriquez JA, Haas CN, Jacangelo J, Gerba CP. Chlorine Inactivation of Adenovirus Type 40 and Feline Calicivirus. Appl Environ Microbiol. 2003;69(7):3979–3985. doi: 10.1128/AEM.69.7.3979-3985.2003 |
| 60. | Dee S, Otake S, Deen J. Use of a Production Region Model to Assess the Efficacy of Various Air Filtration Systems for Preventing Airborne Transmission of Porcine Reproductive and Respiratory Syndrome Virus and Mycoplasma Hyopneumoniae: Results from a 2-Year Study. Virus Res. 2010;154(1–2):177–184. doi: 10.1016/j.virusres.2010.07.022 |
| 61. | Wood C, Tanner B, Higgins L, Dennis J, Luempert L. Effectivenes of a steam cleaning unit for disinfection in a veterinary hospital. Am J Vet Res. 2014;75(12):1083–1088. |
| 62. | Cadnum JL, Jencson AL, Livingston SH, et al. Evaluation of an Electrostatic Spray Disinfectant Technology for Rapid Decontamination of Portable Equipment and Large Open Areas in the Era of SARS-CoV-2. Am J Infect Control. 2020;48(8):951–954. doi: 10.1016/j.ajic.2020.06.002 |
| 63. | Hubrecht R. Comfortable Quarters for Dogs in Research Institutions. In: Reinhardt V, ed. Comfortable Quarters for Laboratory Animals. 9th ed. 2002:57–62. |
| 64. | Eagan BH, Gordon E, Fraser D. The Effect of Animal Shelter Sound on Cat Behaviour and Welfare. Anim Welf. 2021;30(4):431–440. doi: 10.7120/09627286.30.4.006 |
| 65. | Stella J, Croney C, Buffington T. Environmental Factors that Affect the Behavior and Welfare of Domestic Cats (Felis Silvestris Catus) Housed in Cages. Appl Anim Behav Sci. 2014;160(1):94–105. doi: 10.1016/j.applanim.2014.08.006 |
| 66. | Coppola CCL, Enns RM, Grandin T, et al. Noise in the Animal Shelter Environment: Building Design and the Effects of Daily Noise Exposure. J Appl Anim Welf Sci. 2006;9(1):1–7. doi: 10.1207/s15327604jaws0901 |
| 67. | Amaya V, Paterson MBA, Descovich K, Phillips CJC, Au CJCP. Effects of Olfactory and Auditory Enrichment on Heart Rate Variability in Shelter Dogs. 2020;10(8):1385. doi: 10.3390/ani10081385 |
| 68. | Janeczko S, Miller L, Zawistowski S. Canine Housing and Husbandry for Behavioral Well-Being. In: DiGangi B, Cussen VA, Reid PJ, Collins KA, eds. Animal Behavior for Shelter Veterinarians and Staff. 2nd ed. Hoboken: Wiley Blackwell; 2022:236–262. |
| 69. | Wells DL, Hepper PG. A Note on the Influence of Visual Conspecific Contact on the Behaviour of Sheltered Dogs. Appl Anim Behav Sci. 1998;60(1):83–88. doi: 10.1016/S0168-1591(98)00146-4 |
| 70. | Martin AL, Walthers CM, Pattillo MJ, Catchpole JA, Mitchell LN, Dowling EW. Impact of Visual Barrier Removal on the Behavior of Shelter-Housed Dogs. J Appl Anim Welf Sci. 2022:1–11. doi: 10.1080/10888705.2021.2021407 |
| 71. | Pollard V, Shoults A. The Fear Free Design Movement. In: Practical Guide to Veterinary Hospital Design: From Renovations to New Builds. Lakewood, CO: AAHA Press; 2018:51–55. |
| 72. | Boubekri M, Cheung IN, Reid KJ, Wang CH, Zee PC. Impact of Windows and Daylight Exposure on Overall Health and Sleep Quality of Office Workers: A Case-Control Pilot Study. J Clin Sleep Med. 2014;10(6):603–611. doi: 10.5664/jcsm.3780 |
| 73. | UC Davis Koret Shelter Medicine Program. Shelter Intake and Pathway Planning. Information Sheet: Shelter Design and Housing. 2021. Accessed Dec 13, 2022. https://www.sheltermedicine.com/library/resources/?r=shelter-intake-and-pathway-planning. |
| 74. | Taylor S, Denis KS, Collins S, et al. 2022 ISFM/AAFP Cat Friendly Veterinary Environment Guidelines. J Feline Med Surger. 2022;24(11):1133–1163. doi: 10.1177/1098612X221128763 |
| 75. | Hurley KF, Miller L. Introduction to Disease Management in Animal Shelters. In: Miller L, Hurley K, eds. Infectious Disease Management in Animal Shelters. 1st ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2009:5–16. |
5. Sanitation
5.1 General
Maintaining a sanitary environment is an integral part of supporting health and welfare and minimizing the risk of infectious disease. Whether or not infectious disease occurs is dependent on the interaction of several factors: the animal (e.g. species, age, and immunity), the pathogen (e.g. infectious dose and ability to survive outside of the body), and the environment (e.g. temperature, housing, and amount of pathogens present), and how each of these factors are managed1 (Fig. 5.1).
Figure. 5.1. Factors impacting disease transmission in the shelter.
Through cleaning and the proper use of disinfectants, the number of pathogens in the environment is reduced, decreasing the likelihood of spread.2 A clean shelter increases the comfort level of the animals and personnel, and presents a positive image of the shelter to the public.3,4 Protocols for proper sanitation are essential for any sheltering program.
5.2 Definitions
Cleaning is defined as the manual removal of urine, fecal matter, food waste, hair, bodily fluids, and other debris from the environment.2,4,5 Oils and grime found on surfaces, especially soiled, porous, or rough surfaces, can interfere with the ability to kill pathogens6 (see Appendix E). Detergents and degreasers break down oil and grime with soap-like action and can remove up to 90% of environmental pathogens.3,7–9
Disinfection, typically by the application of a chemical product to a clean surface for a specific time period, is the process of killing most of the remaining pathogens.9 Sanitation refers to the combination of cleaning and disinfection. Cleaning and disinfection are separate steps, even when using a detergent-disinfectant combination product that is labeled for both purposes.2
Sterilization is the destruction of all pathogens (e.g. viruses, bacteria, and fungi), including spores, and is generally reserved for surgical instruments and other equipment necessary for sterile procedures.9 True sterilization of cage and kennel surfaces does not occur.
5.3 Sanitation practices
Shelters must have a sanitation plan for all locations in which animals are present, including enclosures, common-use areas, foster homes, and outdoor spaces. Sanitation protocols are used to describe which areas to sanitize, which products to use, and how to use them.4
Sanitation protocols should be based on pathogens, routes, and risk of transmission. Sanitation protocols must include steps for removal of organic matter, cleaning, and disinfection.4 Ideally, sanitation protocols will be developed in consultation with a veterinarian experienced in shelter medicine.4 Those making decisions about sanitation protocols need to be familiar with the active ingredients of common disinfectants, target pathogens, and potential routes of transmission. An increasing number of resources provide guidelines tailored to the shelter environment.6,10,11
Sanitation products must be diluted and used according to label instructions or published recommendations. Solutions that are too weak may be ineffective, and those that are too strong may be harmful to animals and people.4,9 Some disinfectants such as quaternary ammonium products and bleach can be harmful when animals contact or ingest them, even at recommended dilutions, so removing the residue is an essential step.3,4
Disinfectants used in animal areas must be effective against non-enveloped viruses, such as parvovirus, panleukopenia, and calicivirus. Several studies have found that quaternary ammonium-based products, which are commonly used in shelters and veterinary clinics, do not eliminate non-enveloped viruses in spite of label claims.12–15 Other products, such as accelerated hydrogen peroxide, potassium peroxymonosulfate, and bleach products, are effective against non-enveloped pathogens and dermatophytes at the appropriate concentration and contact time.2,12–15
Adequate sanitation cannot be accomplished by using water alone, by spraying and quickly wiping off a disinfectant, or by using a disinfectant with no detergent properties (i.e. bleach) without cleaning first.2,4 Alternative methods of disinfection such as ultraviolet light, steam, freezing, and air filtration systems must not be relied on as the sole means of sanitation in shelters.9,16–24
Sufficient personnel must be assigned to complete sanitation tasks promptly each day so that animals spend most of their time in sanitary conditions. Industry guidelines recommend a minimum of 9 min per animal per day for routine cleaning of enclosures.25 The actual time needed to accomplish daily sanitation will vary based on population, housing size and type, specific products and protocols, and facility use. Calculating how long proper sanitation typically takes per housing unit can provide better estimates of sanitation staffing needs in individual shelters (see Population Management).
Sanitation should proceed in an order that minimizes both the risk of pathogen transmission from infected animals and the exposure of vulnerable animals. In general, the recommended order of cleaning and care, from first to last, is:
- healthy puppies and kittens
- healthy adult animals
- unhealthy animals3
This order of cleaning may be customized to include specific animals or subpopulations (e.g. different infectious diseases and immune-compromised animals) based on the specific needs of the shelter, population, and protocols.5,26
Sanitation practices should be observed regularly to ensure consistency with written protocols. Observation of sanitation practices provides an opportunity to identify and correct deviations from the protocols.3 It is important to ensure that contact times are observed, supplies are readily available, and equipment is adequate for the job.
Pathogen risks in a shelter can change over time, and shelters may need to alter sanitation protocols when disease rates increase or a more difficult to kill pathogen is identified. During an outbreak, protocols should be reviewed and practices observed to ensure efficacy against suspected pathogens.11,27 Pathogens can be spread inadvertently when protocols are improper or practices are not in line with protocols. Common mistakes include incorrect choice of disinfectant, under or over-dilution, not observing contact times, etc.28,29
5.3.1 Sanitizing primary enclosures
Sanitizing primary enclosures is critical to ensure health and comfort. Enclosures must be completely sanitized before being occupied by a different animal.4 This process, also known as deep cleaning, is important even if an animal has only occupied a primary enclosure for a short period of time, the enclosure is not visibly soiled, or the animal appears healthy. Animals are capable of shedding pathogens without showing signs of illness.30 Sanitation is indicated when enclosures are heavily soiled, an infectious disease is diagnosed and on a regular schedule based on use. Table 5.1 shows basic steps and indications for sanitation of primary enclosures.
Sanitation methods significantly impact animal health and welfare. Splattering or soaking animals when spraying water, cleaning, or disinfection products can cause significant distress. It is unacceptable to spray primary enclosures while animals are inside them.3,4,31 Animals need to be removed from nearby housing compartments when overspray is likely.
Adequate drainage is essential for animal housing areas regularly hosed or sprayed with cleaning fluids.32,33 Drainage systems or operational practices (e.g. squeegee and towel drying) must prevent the accumulation of standing water. Dry surfaces are required before animal use because they promote animal comfort and drying aids in the inactivation of pathogens.
Ideally, mopping is avoided in animal housing areas. Mops may harbor pathogens, allowing them to be deposited in other locations.4 However, mopping may be necessary when sanitizing animal enclosures and ward hallways that do not have drains. When mopping cannot be avoided, personnel must ensure that both cleaning and disinfection of the floor surface occur. Mop heads require disposal or sanitation and drying between uses, including between cleaning and disinfection products and between housing areas.
5.3.2 Spot cleaning primary enclosures
When an animal will remain in their enclosure and it has not been heavily soiled, complete sanitation of the enclosure may not be necessary or supportive of animal health.3,4,34,35 Daily cleaning is essential, even in cage-free or home environments, but can often be accomplished using a spot cleaning method.
During spot cleaning, an animal may remain in their enclosure or be given out-of-kennel enrichment. Multi-compartment enclosures facilitate spot-cleaning by allowing personnel to clean in the other compartment to avoid animal contact. Spot cleaning should be conducted at least daily when an animal will remain in the same enclosure. Soiled bedding, old food, urine, and feces are removed, the area tidied, and food and water resupplied (Table 5.1).
Spot cleaning is typically less stressful for animals as it requires less animal handling and does not remove familiar scents.36 Spot cleaning is particularly important for shy or under-socialized animals, and animals with mild diseases worsened by stress (e.g. feline infectious respiratory disease).
5.4 Reducing pathogen spread
Fomites are objects that may be contaminated with pathogens and contribute to transmission of disease. Hands, work clothing, medical equipment, food bowls, litter boxes, toys, and cleaning and handling equipment may serve as fomites.4 Care to avoid the spread of disease through fomites is important during sanitation and when interacting with animals in the shelter.
5.4.1 Personal protective equipment
Personal protective equipment (PPE) is a physical barrier that reduces the spread of disease when used properly. PPE should be selected based on specific pathogens and exposure risks within each population (see Public Health). As the health of the population varies, the type of protective equipment needed may also vary. Appropriate PPE should be used in each area and disposed of or sanitized before proceeding to care for other animals37 (Appendix C).
PPE may need to be changed between individual enclosures or areas based on disease risk because contaminated PPE can contribute to pathogen spread. Protective garments must be changed between handling each animal when there is a high risk for disease transmission.38 Staff training, adequate supplies, and facility set-up (e.g. location of trash receptacles) allow for proper use and removal of PPE. Personnel should wash hands after removing PPE.
5.4.2 Hand hygiene
Hand sanitation is a key part of preventing disease transmission.37,39 Hand hygiene stations should be available in or near every area where contact with animals occurs.40 Ideally, hand hygiene stations are sinks that allow washing with soap and water, and drying with single use towels. At a minimum, hand hygiene stations provide hand sanitizer with at least 60% alcohol.41 Because hand sanitizers are ineffective against some of the most concerning pathogens in shelters (e.g. parvovirus, calicivirus, and ringworm), hand sanitizers should not be relied on as the sole means of hand hygiene.41,42
Proper handwashing technique includes wetting hands with clean, running water; applying and scrubbing with soap for at least 20 seconds; rinsing with clean water; and drying thoroughly with a fresh towel or forced air.43 Proper hand sanitizer techniques include applying 1–2 pumps of gel product to one hand and then rubbing hands together until all surfaces are covered and dry (approximately 20 seconds). Hand sanitizer should only be used on hands that are visibly clean.41
Sanitation protocols must address hand hygiene for shelter staff, volunteers, and visitors.3,4,37 Although all people can move pathogens around, shelter personnel are significantly more likely to do this while they complete daily care tasks compared to shelter visitors.44
5.4.3 Equipment and supplies
All items that come into contact with animals should be sanitized on a regular basis, whenever visibly soiled, and when in direct contact with bodily fluids. In the case of disease outbreaks or when proper sanitation of supplies is not possible between animals, the use of disposable items may be warranted. It is essential to note that gloves, clothes, and shoes can serve as fomites, underscoring the importance of the proper use and replacement of PPE.
Separate cleaning supplies must be designated for each shelter area or be sanitized prior to use in each area. Some supplies need to be changed or sanitized between enclosures, such as rags or towels. Other supplies, such as mop heads and squeegees, can be changed between areas, unless there is a high risk of disease transmission.
Transport cages and traps, as well as vehicle compartments used for animal transport, must be sanitized before being occupied by a different animal.45 Mobile equipment such as rolling trash cans, shopping carts, and food or treatment carts should be assigned to one area or be sanitized between areas.45,46 Sanitation of these items includes wheels and outside contact surfaces. Objects with scratched, damaged, and porous surfaces are difficult or impossible to completely disinfect and should be used with caution or discarded between animals.47 These objects include plastic litter pans, airline carriers, and plastic or unglazed ceramic water bowls.
All bedding and other textiles used at the shelter must be discarded or laundered and thoroughly dried when visibly soiled and before reuse with a different animal.45 Items that are heavily soiled may need to be laundered separately from other textiles.29,48,49 Organic debris (e.g. feces) should be removed from items before laundering.37 Items that cannot be readily disinfected, such as leather gloves and muzzles, may contribute to disease spread when used with animals who appear ill and/or during a disease outbreak.45 Routine cleaning or laundering of bedding could fail to remove non-enveloped viruses and dermatophytes; in these situations, discarding the items in question or using pathogen-specific laundry protocols is recommended.29,49
Automatic watering devices and water bottles should not be used if the watering valve cannot be sanitized before being used by another animal.50,51 Food and water bowls must be sanitized in a different location or at a different time than litter pans or items soiled by feces, to prevent cross contamination.4,52 Dishwashers have excellent mechanical washing action and attain high temperatures which destroy the majority of pathogens but may not destroy non-enveloped viruses such as parvoviruses.26,53 The best way to inactivate these viruses is through the application of a disinfectant to the dishes following the dishwasher cycle. When a dishwasher is not available, disinfectant can be applied following thorough washing and rinsing by hand.52 Basins used to sanitize food and water bowls and litter pans should be thoroughly sanitized between uses.3
5.5 Other shelter areas
Foot traffic plays a role in fomite transmission throughout the shelter and grounds; dedicated boots that can be sanitized or disposable shoe covers should be used in potentially contaminated or protected areas, such as isolation and surgery.4,54,55 Footbaths must not be relied on for infectious disease control in the shelter.4,56,57 This is because achieving adequate contact time is impractical, and the accumulation of organic debris within the bath inactivates many disinfectants. Poorly maintained footbaths create environments that encourage pathogen growth and contribute to disease spread. It is unacceptable for animals to walk through footbaths.3
Animal waste and bodily fluids must be removed from indoor common spaces as soon as possible.5,58 After removal, the area needs to be sanitized properly. Feces must be removed from outdoor areas between animals or groups.59 To reduce parasite egg accumulation in the environment, daily removal of feces is acceptable, although immediate removal is preferred.
Outdoor areas around the shelter must be kept clean, recognizing it is impossible to disinfect gravel, dirt, and grass surfaces.29 Surface covers (e.g. pea gravel, mulch, and rubber chips) can be replaced or recovered regularly to reduce contaminant load. To manage this risk, many shelters designate certain outdoor areas for use by specific animals. This allows closure of an area when needed while still preserving other areas for continued use. Access to areas that cannot be sanitized should be restricted to adult animals who have been vaccinated, dewormed, and appear healthy, or animals for whom the benefits of such access outweigh the risks of disease exposure or transmission.60,61
Standing water should not be allowed to accumulate in or around the shelter because mosquitos breed and many pathogens thrive in wet environments.62,63 Well drained substrates and exposure to sunlight aid in the destruction of pathogens; however, some pathogens survive even in environmental extremes.
5.6 Wildlife, rodent, and insect control
Rodents and insects may harbor pathogens that can spread to shelter animals through direct ingestion, contamination of pet food, or contamination of the environment. Areas of food storage are particularly vulnerable to infestation. All food must be protected from wildlife, rodents, and insects.64,65 Properly storing food bags in sealed bins, promptly cleaning spills or waste, and resealing and refrigerating opened food containers (animal or human) can help mitigate infestations. Rodent and insect control solutions must be safe, humane, and effective.66 Integrated pest management plans are recommended and utilize a variety of environmental measures to reduce the need for pesticides, rodenticides, and insecticides.67
References
| 1. | Ahrens W, Krickeberg K, Pigeot I. An Introduction to Epidemiology. In: Ahrens W, Pigeot I, eds. Handbook of Epidemiology. 2nd ed. New York, NY: Springer Science and Business Media LLC; 2015:3–13. |
| 2. | Weese JS. 14: cleaning and Disinfection. In: Sykes JE, ed. Greene’s Infectious Diseases of the Dog and Cat. 5th ed. Amsterdam: Elsevier; 2022:162–169. |
| 3. | Steneroden K. Sanitation. In: Miller L, Zawistowski S, eds. Shelter Medicine for Veterinarians and Staff. 2nd ed. Ames, IA: Wiley Blackwell; 2013:37–47. |
| 4. | Karsten CL. Sanitation. In: Miller L, Janeczko S, Hurley KF, eds. Infectious Disease Management in Animal Shelters. 2nd ed. Hoboken, NJ: Wiley Blacklwell; 2021:166–190. |
| 5. | Smith M, American Humane. Operational Guide: Sanitation and Disease Control in the Shelter Environment. 2010. Accessed Dec 13, 2022. http://unddr.org/uploads/documents/OperationalGuide.pdf |
| 6. | Dvorak G, Roth J, Amass S. Disinfection 101. Accessed Dec 13, 2022. www.cfsph.iastate.edu |
| 7. | Russell A, Huge W. Chemical Disinfectants. In: Linton AH, Huge WB, Russell AD, eds. Disinfection in Veterinary and Farm Animal Practice. Oxford: Blackwell Scientific Publications; 1987:12–42. |
| 8. | Morgan-Jones S. Practical Aspects of Disinfection and Infection Control. In: Linton A, Hugo W, Russel A, eds. Disinfection in Veterinary and Farm Animal Practice. Oxford: Blackwell Scientific Publications; 1987. |
| 9. | Rutala WA, Weber DJ. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008: update May 2019. Centers for Disease Control and Prevention, Department of Health and Human Services; 2020:8–163. |
| 10. | DiGangi BA, Kommedal AT. Sanitation and Surgical Asepsis. In: Polak KC, Kommendal AT, eds. Field Manual for Small Animal Medicine. First. Hoboken, NJ: Wiley-Blackwell; 2018:263–288. |
| 11. | Dvorak G, Rovid Spickler A. Disinfection 101. In: Peterson C, Dvorak G, Rovid Spickler A, eds. Maddie’s Infection Control Manual for Animal Shelters for Veterinary Personnel. Ames, IA: Iowa State University, Center for Food Security and Public Health; 2008:42–64. |
| 12. | Eleraky NZ, Potgieter LND, Kennedy MA. Virucidal Efficacy of Four New Disinfectants. J Am Anim Hosp Assoc. 2002;38(3):231–234. doi: 10.5326/0380231 |
| 13. | Moriello KA, Deboer DJ, Volk LM, Sparkes A, Robinson A. Development of an In Vitro, Isolated, Infected Spore Testing Model for Disinfectant Testing of Microsporum Canis Isolates. Vet Dermatol. 2004;15(3):175–180. doi: 10.1111/j.1365-3164.2004.00390.x |
| 14. | Scott F. Virucidal Disinfectants and Feline Viruses. Am J Vet Res. 1980;41:410–414. doi: 10.1017/CBO9781107415324.004 |
| 15. | Kennedy M, Mellon V, Caldwell G, Potgieter LND. Virucidal Efficacy of the Newer Quaternary Amonium Compounds. J Am Anim Hosp Assoc. 1995;31(3):254–258. |
| 16. | Pearce-Walker JI, Troup DJ, Ives R, et al. Investigation of the Effects of an Ultraviolet Germicidal Irradiation System on Concentrations of Aerosolized Surrogates for Common Veterinary Pathogen. Am J Vet Res. 2020;81(6):506–513. doi: 10.2460/ajvr.81.6.506 |
| 17. | Cadnum JL, Jencson AL, Livingston SH, et al. Evaluation of an Electrostatic Spray Disinfectant Technology for Rapid Decontamination of Portable Equipment and Large Open Areas in the Era of SARS-CoV-2. Am J Infect Control. 2020;48(8):951–954. doi: 10.1016/j.ajic.2020.06.002 |
| 18. | Tomb RM, Maclean M, Coia JE, et al. New Proof-of-Concept in Viral Inactivation: Virucidal Efficacy of 405 nm Light Against Feline Calicivirus as a Model for Norovirus Decontamination. Food Environ Virol. 2017;9(2):159–167. doi: 10.1007/s12560-016-9275-z |
| 19. | Nuanualsuwan S, Mariam T, Himathongkham S, Cliver DO. Ultraviolet Inactivation of Feline Calicivirus, Human Enteric Viruses and Coliphages. Photochem Photobiol. 2002;76(4): 406–410. doi: 10.1562/0031-8655(2002)076<0406:uiofch>2.0.co;2 |
| 20. | Department of Human Health Services. Enforcement Policy for Sterilizers, Disinfectant Devices, and Air Purifiers during the Coronavirus Disease 2019 (COVID-19) Public Health Emergency. 2020. Accessed Dec 13, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents |
| 21. | Kim D, Kang D. UVC LED Irradiation Effectively Inactivates Aerosolized Viruses. Appl Environ Microbiol. 2018;84(17):1–11. |
| 22. | Thurston-Enriquez JA, Haas CN, Jacangelo J, Gerba CP. Chlorine Inactivation of Adenovirus Type 40 and Feline Calicivirus. Appl Environ Microbiol. 2003;69(7):3979–3985. doi: 10.1128/AEM.69.7.3979-3985.2003 |
| 23. | Dee S, Otake S, Deen J. Use of a Production Region Model to Assess the Efficacy of Various Air Filtration Systems for Preventing Airborne Transmission of Porcine Reproductive and Respiratory Syndrome Virus and Mycoplasma Hyopneumoniae: Results from a 2-Year Study. Virus Res. 2010;154(1–2):177–184. doi: 10.1016/j.virusres.2010.07.022 |
| 24. | Wood C, Tanner B, Higgins L, Dennis J, Luempert L. Effectivenes of a Steam Cleaning Unit for Disinfection in a Veterinary Hospital. Am J Vet Res. 2014;75(12):1083–1088. |
| 25. | National Animal Care and Control Association. NACA Guidelines. National Animal Care and Control Association, ed. Murrietta, CA: NACA Board of Directors; 2014. |
| 26. | Gilman N. Sanitation in the Animal Shelter. In: Miller L, Zawistowski SL, eds. Shelter Medicine for Veterinarians and Staff. Ames, IA: Blackwell; 2004:67–78. |
| 27. | O’Quin. J. Outbreak Management. In: Miller L, Zawistowski S, eds. Shelter Medicine for Veterinarians and Staff. 2nd ed. Ames, IA: Wiley Blacklwell; 2013:349–370. |
| 28. | Miller L, Hurley K, Dvorak G, Petersen C. Sanitation and Disinfection. In: Miller L, Hurley K, eds. Infectious Disease Management in Animal Shelters. Ames, IA: Wiley-Blackwell; 2009:49–60. |
| 29. | Petersen C, Dvorak G, Spickler AR, eds. Maddie’s Infection Control Manual. Ames, IA: Iowa State University Center for Food Security and Public Health; 2008. |
| 30. | Lavan R, Knesl O. Prevalence of Canine Infectious Respiratory Pathogens in Asymptomatic Dogs Presented at US Animal Shelters. J Small Anim Pract. 2015;56:572–576. doi: 10.1111/jsap.12389 |
| 31. | Miller L, Zawistowski S. Housing, Husbandry, and Behavior of Dogs in Animal Shelters. In: Weiss E, Mohan-Gibbons H, Zawistowski S, eds. Animal Behavior for Shelter Veterinarians and Staff. Ames, IA: John Wiley & Sons, Inc.; 2015:145–159. |
| 32. | Schlaffer L, Bonacci P. Shelter Design. In: Miller L, Zawistowski S, eds. Shelter Medicine for Veterinarians and Staff. 2nd ed. Ames, IA: Wiley Blackwell; 2013:21–35. |
| 33. | Pollard V, Shoults A. The Fear Free Design Movement. In: Practical Guide to Veterinary Hospital Design: From Renovations to New Builds. Lakewood, CO: AAHA Press; 2018:51–55. |
| 34. | UC Davis Koret Shelter Medicine Program. Spot Cleaning Cat Cages. Accessed Oct 29, 2020. https://www.sheltermedicine.com/library/resources/?r=spot-cleaning-cat-cages#:~:text=Spot cleaning is a method,and handling cats during cleaning. Published 2015. |
| 35. | Allen MC. Spot-Cleaning Cat Cages. Animal Sheltering Magazine. Accessed Oct 29, 2020. https://www.animalsheltering.org/magazine/articles/spot-cleaning-cat-cages. |
| 36. | Patronek GJ, Lacroix CA. Developing an Ethic for the Handling, Restraint, and Discipline of Companion Animals in Veterinary Practice. J Am Vet Med Assoc. 2001;218(4):514–517. doi: 10.2460/javma.2001.218.514 |
| 37. | Stull JW, Bjorvik E, Bub J, Dvorak G, Petersen C, Troyer HL. 2018 AAHA Infection Control, Prevention, and Biosecurity Guidelines. J Am Anim Hosp Assoc. 2018;54(6):297–326. doi: 10.5326/JAAHA-MS-6903 |
| 38. | Center for Disease Control and Prevention. Personal Protective Equipment (PPE): Coaching and Training Frontline Health Care Professionals. 2018:1–45. Accessed Dec 13, 2022. https://www.cdc.gov/infectioncontrol/pdf/strive/PPE103-508.pdf. |
| 39. | Mathur P. Hand Hygine: Back to the Basics of Infection Control. Indian J Med Res. 2011;134(5):611–620. |
| 40. | The National Association fof State Public Health Veterinrians Animal Contact Compendium Committee. Public Health Compendium of Measures to Prevent Disease Associated with Animals in Public Settings, 2017. J Am Vet Med Assoc. 2017;251(11):1268–1292. |
| 41. | Centers for Disease Control and Prevention. When & How to Use Hand Sanitizer in Community Settings. 2020. Accessed Dec 13, 2022. https://www.cdc.gov/handwashing/show-me-the-science-hand-sanitizer.html |
| 42. | Liu P, Yuen Y, Hsiao HM, Jaykus LA, Moe C. Effectiveness of Liquid Soap and Hand Sanitizer against Norwalk Virus on Contaminated Hands. Appl Environ Microbiol. 2010;76(2):394–399. doi: 10.1128/AEM.01729-09 |
| 43. | Centers for Disease Control and Prevention. When and How to Wash Your Hands. 2022. Accessed Dec 13, 2022. https://www.cdc.gov/handwashing/when-how-handwashing.html |
| 44. | Aziz M. Looking for a Reference or Source for the Recommendation of Allowing the Public to Pet Shelter Animals While They Are in Their Cages or Runs. Question. 2015. Accessed Dec 13, 2022. https://www.sheltermedicine.com/library/resources/?r=looking-for-a-reference-or-source-for-the-recommendation-of-allowing-the-public-to-pet-shelter-animals-while-they-are-in-their-cages-or-runs. |
| 45. | Boone SA, Gerba CP. Significance of Fomites in the Spread of Respiratory and Enteric Viral Disease. Appl Environ Microbiol. 2007;73(6):1687–1696. doi: 10.1128/AEM.02051-06 |
| 46. | Blenkharn J. Potential Compromise of Hospital Hygiene by Clinical Waste Carts. J Hosp Infect. 2006;63(4):423–427. doi: 10.1016/j.jhin.2006.03.002 |
| 47. | Latorre AA, Van Kessel JS, Karns JS, et al. Biofilm in Milking Equipment on a Dairy Farm as a Potential Source of Bulk Tank Milk Contamination with Listeria Monocytogenes. J Dairy Sci. 2010;93(6):2792–2802. doi: 10.3168/jds.2009-2717 |
| 48. | Moriello KA. Decontamination of Carpet Exposed to Microsporum Canis Hairs and Spores. J Feline Med Surg. 2017;19(4):435–439. doi: 10.1177/1098612X16634390 |
| 49. | Moriello KA. Decontamination of Laundry Exposed to Microsporum Canis Hairs and Spores. J Feline Med Surg. 2017;19(4):435–439. doi: 10.1177/1098612X16634390 |
| 50. | Costello T, Watkins L, Straign M, Bean W, Toth LA, Rehg JE. Effectiveness of Rack Sanitation Procedures for Elimination of Bacteria from Automatic Watering Manifolds. Contemp Top Lab Anim Sci. 1998;37(2):50-x1. |
| 51. | Macy JD, Cameron GA, Ellis SL, Hill EA, Compton SR. Assessment of Static Isolator Cages with Automatic Watering when Used with Conventional Husbandry Techniques as a Factor in the Transmission of Mouse Hepatitis Virus. Contemp Top Lab Anim Sci. 2002;41(4):30–35. |
| 52. | Weese JS, Rousseau J. Survival of Salmonella Copenhagen in Food Bowls Following Contanimation with Experimentally Inoculated Raw Meat: effects of Time, Cleaning, and Disinfection. Can Vet J. 2006;47(9):887–889. |
| 53. | Lawler D. Prevention and Management of Infection in Kennels. In: Greene C, ed. Infectious Diseases of the Dog and Cat. 3rd ed. St. Louis, MO: W.B. Saunders; 2006:1046–1051. |
| 54. | Morley P, Morris N, Hyatt D, Van Metre D. Evaluation of the Efficacy of Disinfectant Footbaths as Used in Veterinary Hospitals. J Am Vet Med Assoc. 2005;226(12):2053–2058. doi: 10.2460/javma.2005.226.2053 |
| 55. | Stockton K, Morley P, Hyatt D, et al. Evaluation of the Effects of Footwear Hygiene Protocols on Nonspecific Bacterial Contamination of Floor Surfaces in an Equine Hospital. J Am Vet Med Assoc. 2006;228(7):1068. doi: 10.2460/javma.228.7.1068 |
| 56. | Amass SF, Abvp D, Vlwerberg BD, Ragland D, Dowell CA, Anderson CD. Evaluating the Efficacy of Boot Baths in Biosecurier Protocols. Swine Heal Prod. 2000;8(4):169–173. |
| 57. | Amass S, Arighi M, Kinyon J, Hoffman L, Schneider J, Draper D. Effectiveness of Using a Mat Filled with a Peroxygen Disinfectant to Minimize Shoe Sole Contamination in a Veterinary Hospital. J Am Vet Med Assoc. 2006;228(9):1391–1396. doi: 10.2460/javma.228.9.1391 |
| 58. | Committee NA of SPHVVIC. Compendium of Veterinary Standard Precautions for Zoonotic Disease Prevention in Veterinary Personnel. J Am Vet Med Assoc. 2015;247(11):1252–1265. doi: 10.2460/javma.247.11.1252 |
| 59. | Avcioglu H, Balkaya I. The Relationship of Public Park Accessibility to Dogs to the Presence of Toxocara Species Ova in the Soil. Vector-Borne Zoonotic Dis. 2011;11(2):177–180. doi: 10.1089/vbz.2009.0244 |
| 60. | Bugg RJ, Robertson ID, Elliot AD, Thompson RCA. Gastrointestinal Parasites of Urban Dogs in Perth, Western Australia. Vet J. 1999;157(3):295–301. doi: 10.1053/tvjl.1998.0327 |
| 61. | Schultz RD, Thiel B, Mukhtar E, Sharp P, Larson LJ. Age and Long-Term Protective Immunity in Dogs and Cats. J Comp Patho l. 2010;142(1):S102–S108. doi: 10.1016/j.jcpa.2009.10.009 |
| 62. | Kronenwetter-Koepel TA, Meece JK, Miller CA, Reed KD. Surveillance of Above- and Below-Ground Mosquito Breeding Habitats in a Rural Midwestern Community: Baseline Data for Larvicidal Control Measures against West Nile Virus Vectors. Clin Med Res. 2005;3(1):3–12. doi: 10.3121/cmr.3.1.3 |
| 63. | Stockwell PJ, Wessell N, Reed DR, et al. A Field Evaluation of Four Larval Mosquito Control Methods in Urban Catch Basins. J Am Mosq Control Assoc. 2006;22(4):666–671. doi: 10.2987/8756-971X(2006)22[666:AFEOFL]2.0.CO;2 |
| 64. | New Zealand Ministry for Primary Industries: Regulation and Assurance Branch. Code of Welfare: Dogs. 2018:1–45. Accessed Dec 13, 2022. https://www.agriculture.govt.nz/dmsdocument/1445-pigs-animal-welfare-code-of-welfare |
| 65. | Urban JE, Broce A. Flies and Their Bacterial Loads in Greyhound Dog Kennels in Kansas. Curr Microbiol. 1998;36(3):164–170. doi: 10.1007/PL00006761 |
| 66. | Mason G, Littin KE. The Humaneness of Rodent Pest Control. Anim Welf. 2003;12(1):1–37. |
| 67. | Environmental Protection Agency. Integrated Pest Management Tools: Resources to Support IPM Implementation. 2021. Accessed Dec 13, 2022. https://www.epa.gov/ipm/integrated-pest-management-tools-resources-support-ipm-implementation. |
6. Medical Health
6.1 General
Comprehensive shelter medical programs are the foundation of humane sheltering. The World Health Organization describes health as a state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity.1 Health care for animals in shelters is a necessity and must include attention to overall well-being.2,3
Shelter medical care must begin at or before intake and continue throughout the shelter stay.4–6 Animals may arrive at shelters already experiencing health challenges, while others may develop issues during their stay. When a shelter admits an animal, they become responsible for providing all of the medical and wellness care that the animal needs, or promptly finding an outcome that meets those needs. When medical treatment is necessary, it must be provided in a timely fashion.
Shelters must provide species-appropriate preventive health care; this includes implementing protocols that strengthen resistance to disease and minimize exposure to pathogens, such as vaccination, parasite control, good nutrition, and appropriate handling and housing location.7 Shelters can experience severe disease outbreaks without proactive management, monitoring, and communication.
Individual animal health must be addressed within the balance of decisions and practices that support overall population health. Population health is impacted when spread of disease is likely, when long lengths of stay place the shelter over its capacity for care, and when treatment costs reduce the shelter’s resources to provide care for other animals (see Population Management).
A shelter’s capacity to provide medical care for individual animals is impacted by:
- the availability of resources to safely and humanely provide treatment and maintain welfare during the treatment period
- the duration of care
- the number of animals needing treatment
- likelihood and consequences of disease transmission
- the likelihood of recovery
- and the animal’s potential for a live outcome
Prompt identification and communication of health conditions, and the development of protocols for conditions that are routinely treated or managed by the shelter provide transparency and support timely decision-making. Shelters should have a protocol for making decisions about which animals and conditions to treat, and which animals and conditions they cannot treat.
Tracking disease rates and outcomes for medical cases provides important measures of shelter population health.8 Key indicators of healthcare program deficiencies include the decline of animal health and welfare after intake, sick or injured animals held without prompt treatment, wide-scale disease outbreaks, animals dying or being euthanized as a result of shelter-acquired disease or injury, and chronically high rates of disease. Prevention of disease in shelters through proactive planning of animal pathways (see Population Management) and preventive healthcare supports better animal health and welfare, saves resources, and improves the well-being of shelter personnel.9
6.2 Veterinary oversight and medical recordkeeping
A formal relationship with a veterinarian must be in place to ensure oversight of medical and surgical care in the shelter. Personnel providing medical care must have the skills and equipment to administer prescribed treatments safely and effectively.
Evidence-based protocols are essential for providing a consistent approach to addressing the health of individual animals and populations entering shelters.10 All medical practices and protocols must be developed in consultation with the shelter’s veterinarian (see Management and Record Keeping). Ensuring compliance with healthcare plans and protocols, on a population or individual level, is part of veterinary oversight. In addition to providing details of diagnosis and treatment, shelter medical protocols include instructions for animal housing, sanitation, decision-making, and communication.11 When a medical concern falls outside of standard protocols or does not respond to treatment as expected, a veterinarian must be consulted.
Medications and treatments must only be administered by prescription or in accordance with written protocols provided by a veterinarian.12 Medication should only be prescribed when there exists a reasonable presumptive diagnosis, the ability to administer as directed, and a plan to monitor the course of disease, so that success or failure can be determined.13 Giving medications when not needed, such as prescribing antibiotics to prevent viral infections, can cause harmful side-effects and promote antibiotic resistance.
When drugs are used or dispensed, it must be done in accordance with federal and state regulations.14 These regulations may limit use or dispensing of off-label and compounded drugs. When dispensed or when required by state regulations for in-shelter use, prescription drug labels include:
- name of the prescribing veterinarian
- clinic or shelter name, phone number, and address
- patient identification and species
- date dispensed and expiration date
- drug name, form, and amount
- directions for use
- cautionary statements15
Accurate medical records are an essential part of an animal’s shelter record. A medical history must be requested for all animals presented to the shelter and added to the medical record. Shelters must document all medical care rendered to each animal in the medical record.16 Medical records include accurate identifying information; signalment (age, sex, species, and reproductive status); and a dated list of physical exam findings, vaccinations, diagnostic test results, procedures, and treatments (including medications with dose and route of administration). A record of the animal’s medical care must be provided in hardcopy or electronic form when the animal leaves the shelter’s care.
6.3 Medical assessment
Collecting information about animal health before admission allows the shelter to offer medical services that can prevent intake, such as spay-neuter, outpatient care, or referral to other accessible programs.17 When intake to the shelter is necessary, each animal’s individual health status must be evaluated, documented, and monitored beginning at intake.
Each animal must receive at least a cursory health assessment by trained personnel at intake to check for signs of infectious disease or problems that require emergency medical care.5,18 The intake assessment must include confirmation of the animal’s estimated age, sex, physical description, and the presence of any identification and microchips. Administration of core vaccinations (Table 6.1) and parasite prevention is typically paired with this intake assessment.
A comprehensive physical examination by a veterinarian or trained personnel should also be performed. Ideally, this physical exam is performed within 24 hours of intake. Timely initial assessment and examination allow prompt treatment of medical conditions, establish a health baseline for each animal, and allow recognition of changes in health during the animal’s time in care. Screening tests can be a part of this assessment, including FeLV and FIV testing and management in animal shelter’s policy19 (see ASV Position Statement).20 Findings from any assessments and examinations are documented in the individual animal’s medical record and used to inform housing and flow-through planning.
Animals with signs of infectious disease at intake should be isolated until determined to be low-risk to the population. Separating potentially infectious sick animals reduces the risk of fomite transmission by personnel and prevents spread through shared environments.
Quarantining healthy animals at intake is not generally recommended. Quarantines are appropriate only for animals with a history of direct, high-risk infectious disease exposure. Unnecessary holds increase length of stay and are detrimental to animal health and organizational goals (see Population Management).
Some animals are more susceptible and require greater protection from possible disease exposure. Heightened precautions to prevent disease transmission should be taken when handling more susceptible animals, such as juveniles, older animals, and those with underlying conditions. Precautions typically include placement in foster care, limiting the number of people in contact, using personal protective equipment (PPE), and providing care for the most vulnerable first (Appendix C).
Trained personnel must visually observe the health and well-being of every animal at least once every 24 hours.16 Ideally, daily monitoring observations take place before cleaning, so that food intake and condition of the enclosure, including feces, urine, or vomit, can be noted. Medical staff are essential members of the shelter’s comprehensive care team; a medical staff member should attend population rounds with representatives from other departments (see Population Management).
Animals staying in the shelter long-term require regular medical assessment. At minimum, an examination by trained personnel, including weighing and body condition score, should be repeated on a monthly basis. A comprehensive exam should be performed at least every 6 months while in shelter care, including animals in foster. More frequent examinations are necessary for animals with chronic conditions and when new concerns are observed.
6.4 Essential wellness and preventive care
Prevention and early detection of health concerns in the shelter is critical to supporting physical and emotional well-being. Vaccination, parasite control, proper nutrition, and addressing specific care needs for individual animals improves the health of individuals and populations, while saving the shelter time and resources. For example, grooming and bathing are essential components of animal care and must be provided when necessary for animal health or comfort.11
6.4.1 Vaccination
A timely vaccination program is fundamental to preventing severe disease outbreaks in animal shelters.21,22 Shelters must have a written vaccination protocol developed under the supervision of the shelter’s veterinarian (see Management and Record Keeping). Shelter vaccine protocols differ from protocols used in private practice because shelter animals are subject to an increased risk of infectious disease.11,23 Risk factors include stressors, exposure to other animals, age, previous preventive care, and pathogen levels in the environment.11,24–27 Key differences in protocols compared to those recommended in private practice include an earlier and longer age range for juveniles, a shorter time span between vaccines, and different core and noncore products.11,23
Shelters must properly handle and store vaccines according to manufacturer guidelines. Proper handling includes refrigeration along the supply chain and within the shelter, preventing freezing, reconstitution according to manufacturer instructions, and discarding modified live vaccines reconstituted more than 1 hour prior to use.4,25,27–29 Proper technique for vaccine administration is important for efficacy and safety. This includes use of the dose and route indicated by the manufacturer, a sterile syringe and a fresh needle, and gentle handling.4,28–30 The location for specific vaccine injections should follow administration site guidelines.28,30 Recording the serial and batch number information in the medical record is required for rabies vaccines and is recommended for all vaccines in case of adverse reactions, recalls, or vaccine failures.
Shelters must have protocols for recognizing, managing, and reporting adverse vaccine reactions, and required treatments must be accessible.25,31 This includes protocols for accidental subcutaneous administration of intranasal vaccines, which can lead to significant infection or allergic reactions.4 Management of vaccine reactions might include alerting the veterinarian, close monitoring, administration of medications, or referral to an emergency clinic, depending on the situation and severity of the reaction.27 Vaccine reactions need to be reported to the manufacturer.32
6.4.2 Core vaccines in shelters
A core vaccine is one given to all eligible animals and is withheld only in extraordinary circumstances.27 For all core vaccines except rabies, shelters should use modified live virus or recombinant vaccines (MLV) rather than killed products because they provide a faster immune response.33–35 This includes vaccines for puppies, kittens, animals with FeLV or FIV, and pregnant and nursing animals.30,36 Cerebellar hypoplasia is a theoretical complication of MLV panleukopenia vaccination of pregnant cats; however, the risk of abortion, maternal, and kitten death due to panleukopenia generally outweighs this concern in shelters.37,38
MLV vaccines create effective, long-lasting immunity to distemper-, parvo-, adeno-, and panleukopenia viruses in dogs and cats with competent immune systems within days of initial vaccination and may provide partial protection sooner.33,39,40 MLV vaccines also decrease symptoms and duration of herpes-, calici-, and parainfluenza virus and Bordetella infections.25,34,35,41,42
Dogs
A subcutaneous MLV vaccine for canine distemper-, adeno-, parvo-, and parainfluenza viruses (DAPP) is core for shelter puppies and dogs.21 An intranasal vaccine containing both Bordetella and parainfluenza virus (Bord/PI), with or without adenovirus, is also core for shelter puppies and dogs.21 The intranasal route is important to maximize efficacy and activate respiratory immune cells, which can provide additional protection against other infectious respiratory diseases.43,44
Cats
A subcutaneous MLV vaccine for feline viral rhinotracheitis, calicivirus, and panleukopenia viruses (FVRCP) is core for shelter cats and kittens. Feline intranasal vaccination for herpes and calicivirus has a similar efficacy to the injectable, but there is questionable reliability of intranasal vaccination against panleukopenia virus.23,39 Using both subcutaneous and intranasal vaccines together is safe but has not been shown to increase immunity over either product alone. The intranasal vaccine may provide protection against herpes and calicivirus to young kittens through reduced maternal antibody interference.23
Rabies
Eligible dogs and cats should be vaccinated against rabies before leaving shelter care.11 Rabies vaccines must be administered following state and local guidelines and the most recent Compendium for Animal Rabies Prevention and Control.45–48 Specific regulations for how rabies vaccination is to be documented and who can administer the vaccine vary by state. Puppies and kittens that are too young for rabies vaccination may be adopted or transported with the recommendation that new caretakers provide vaccination when old enough. Rabies vaccination of animals under 12 weeks of age, although considered off-label, appears safe and may be of value in some situations (e.g. return-to-field).49 Feral cats should receive all core vaccines at the time of spay-neuter, regardless of age.50
After the initial series (see Table 6.1), vaccination protocols for animals housed long-term in shelters are best guided by the shelter’s veterinarian.
6.4.3 Noncore vaccines
Noncore vaccines (e.g. Canine influenza, Leptospira, Lyme; Feline Bordetella, Chlamydia, leukemia virus, etc.) may be useful when prescribed by a veterinarian for specific animals, subpopulations, or in the face of diagnosed outbreaks. When deciding whether to use noncore vaccines, it is important to consider the onset of immunity and the number of boosters, as many of these vaccinations may not be fully effective for 10–14 days after the final dose.23
6.4.4 Vaccine schedules
Adult animals must be vaccinated with core vaccines at or before intake (Table 6.1). Revaccination 2–4 weeks later is suggested for those still in shelter care, especially when disease risk is high. Animals housed in shelters should be vaccinated with core vaccines even if ill or pregnant, as the individual and population risks of not vaccinating outweigh the small risk of vaccination.25,30,38 Vaccinating an animal with all core products on the same day or during a surgical procedure does not decrease immune responsiveness to those vaccines or significantly increase the chance of adverse reactions.29,36,51–53
Puppies and kittens housed in shelter facilities must begin core vaccinations at or before intake starting at 4 weeks old and must be revaccinated every 2 weeks until 20 weeks old.4,25,28 Shelter personnel and veterinarians can use dentition, behavior, body weight, and available history to estimate age when date of birth is unknown.54 In juvenile shelter-housed animals, frequent vaccination is critical to ensure that animals develop their own protective antibodies as soon as possible after antibodies provided by their mother wane.28,55 When no longer housed in the shelter facility (i.e. in foster or adopted), juvenile vaccine schedules may be adjusted.
The risk of puppies and kittens contracting and spreading infections such as parvo, distemper, and panleukopenia can be greatly reduced by housing litters in individual foster homes until they are old enough for spay-neuter and adoption. Puppies and kittens housed in foster care must begin core vaccinations at or before intake starting at 4 weeks old and must be revaccinated at the veterinarian’s discretion every 2–4 weeks until 20 weeks old.4,25,28 Assessment of infectious disease risk in the foster home will determine whether a shorter or longer interval is appropriate.
It is not recommended to delay placement outcomes (e.g. adoption and transport) to allow response to vaccination or to receive a booster. The safer alternative is to secure an outcome with the recommendation that new caretakers continue a veterinary-directed vaccination protocol that reflects the animal’s new lifestyle and disease risks.
6.4.5 Parasites
Parasites, both internal and external, are one of the most common health concerns seen in shelter dogs and cats.56 Some animal parasites can also impact human health (e.g. roundworms, hookworms, mites, ticks, and fleas). Animals should receive anti-parasite treatments at or before intake and throughout their shelter stay.
An effective parasite control program, including medications and environmental control, should be designed with the supervision of a veterinarian. Considerations include the impact of the parasite on individual animals, the shelter population, and human health. Because risks vary geographically, it is important to identify the parasites of concern in the shelter and in the community the animal comes from, including those received through relocation programs. Effective protocols tailor treatments to the species and life stage of their animals, including age, pregnancy, and lactation.57–61 For example, treatment for coccidia may be considered for juvenile animals at intake to reduce disease severity and environmental contamination.
All dogs and cats must be treated for roundworms and hookworms at intake, starting at 2 weeks of age, because these organisms can cause harm to people, especially children.62 Parasite treatment also reduces contamination of the shelter environment where animals and humans may be exposed. Since most parasite eggs or cysts are shed in high numbers through feces and are difficult or impossible to kill, feces should be promptly removed from animal housing and exercise areas.63,64 Good sanitation practices, especially mechanical cleaning of soiled areas, reduce the potential for spread.56
Regardless of geographic location, all shelters should have policies regarding testing, prevention, and management of heartworm disease.65–69 This policy may specify in-shelter prevention, treatment and management protocols, or may describe a plan for referral of adopters to local veterinarians for testing or care.
6.4.6 Nutrition
Shelters should seek veterinary input when developing a feeding protocol for their animal population. Food that is consistent with the nutritional needs, health status, and species of the individual animal must be provided at least daily. Food must be fresh, palatable, free from contamination, and not shared between enclosures. Feeding a consistent diet can support animal health and streamline feeding protocols. Fresh, clean water must be available to animals unless there is a medical reason for water to be withheld for a prescribed period of time.
The amount and frequency of feeding vary depending on life stage, species, size, activity level, health status of the animal, and the particular diet chosen. Ideally, healthy adult dogs are fed twice daily, and cats are fed multiple small meals or allowed to forage throughout the day. When managing starved animals or those with unique nutritional needs, veterinary input must be sought. Healthy puppies and kittens as well as lactating and pregnant animals must be fed small amounts frequently or have food available through the day (i.e. free-choice).
Food intake must be monitored daily. Loss of appetite or inability to eat are health concerns that require medical attention. Since animals have highly variable metabolic requirements, each animal should be fed to meet individual needs and prevent excessive gain or loss of body weight.54,70 Body condition and hydration status of animals must be monitored. When animals are cohoused, matching animals with similar nutritional needs or having a process for feeding separately is important. Cohoused animals should be monitored during feeding times, so that appetite and conflicts around food may be addressed.
Food and water dishes must be safe, sufficient in number, and of adequate size. For litters and cohoused adults, providing at least one food dish per animal is recommended. Distributing dishes throughout the enclosure can help prevent guarding behavior (see Facilities).
Supplies of food must be stored in a manner to prevent spoilage or contamination, including refrigeration for perishable foods. Food waste creates a health hazard through spoilage and attraction of pests.
6.4.7 Pregnant, nursing, and neonatal animals
Shelters should have a protocol for the care of pregnant, nursing, and neonatal animals.71 This includes whether or not an animal will be spayed or allowed to go to term (see Surgery). Shelters housing pregnant, nursing, or neonatal animals must ensure that additional disease prevention, nutrition, and stress reduction measures are taken, to protect these vulnerable populations. Housing pregnant and nursing animals in foster care provides significant medical and behavioral benefits, including minimizing risk of infectious disease transmission and facilitating more consistent monitoring. Pregnant and neonatal animals may require urgent interventions, so protocols for accessing emergency care, additional training, and resources are needed to support these populations.
6.5 Responding to health concerns
Any animal observed to be experiencing pain, suffering, or distress; rapidly deteriorating health; life-threatening problems; or suspected zoonotic medical conditions must be promptly assessed and managed.16 Communication is a key part of facilitating care. Protocols for documenting and reporting health concerns are essential.
Protocols for common diseases and health conditions, which specify diagnostics, medical care, and management (e.g. housing, PPE, and outcomes) are an integral part of any shelter health program. Infectious disease protocols must include measures both to minimize transmission and to ensure appropriate care of the infected animals. The response to each disease will look different for every organization, due to the variety of pathogens encountered, modes of transmission, and types of facilities. The shelter veterinarian should be consulted on all policies and protocols related to the maintenance of medical and behavioral animal health (see Management and Record Keeping).
6.5.1 Pain management
Animals with acute or chronically painful medical conditions are often cared for by shelters. Pain must be recognized and treated to alleviate suffering. Treatment of pain can include providing euthanasia. Unrelieved pain is a significant welfare concern and can result in chronic physical manifestations, such as weight loss, muscle breakdown, increased blood pressure, and prolonged recovery from illness or injury, as well as mental and emotional distress.72 Failure to provide treatment for pain is unacceptable.
Recognizing and alleviating pain in a wide variety of species can be complex and difficult.73 Individual animals react differently to painful stimuli and may show a variety of clinical and behavioral signs.2 Observation of behavior and knowledge of the causes of pain are the most accurate ways of assessing pain in animals; if a procedure, injury, or condition is known to be painful in humans, it can be assumed to be painful in animals. Several published scales are available to assess pain in animals.74 When an animal is suspected to be painful, it is the responsibility of shelter staff to follow veterinary protocols and request veterinary assessment.
Protocols for the treatment of painful conditions should be created by a veterinarian. Pain control provided must be of an appropriate strength and duration to preempt or relieve pain. When pain can be anticipated, as with surgical procedures, pain control should be provided before the painful event. The use of controlled drugs must be supervised by a veterinarian as required by regulatory statutes.
Non-pharmacological approaches to pain (e.g. the presence of littermates, a quiet environment, massage, physical therapy, heat, and deep bedding) can supplement pharmacologic interventions to help increase comfort and alleviate anxiety.
Animals must be reassessed frequently to determine the efficacy of pain relief provided. When the pain relief provided is inadequate, emergency medical care must be provided.
6.5.2 Emergency medical care
An emergency medical plan must be in place to provide appropriate and timely veterinary care for any animal who is injured, in distress, or showing signs of significant illness.16 The emergency medical plan must indicate how staff will recognize and report medical conditions requiring emergency care. The emergency medical plan should specify whether emergency services are provided on site or through an outside veterinary clinic. Animals housed outside the shelter facility (e.g. in foster or off-site adoption centers) are subject to the same guidance. Foster care providers should be given clear instructions about how and when to access emergency and after-hours care.
If the emergency medical plan cannot be implemented or fails to relieve suffering, the animal should be euthanized.16 Many shelters care for animals they do not legally own, such as those impounded as strays, held as evidence in legal cases, or boarded for owners requiring temporary assistance. Agreements between the shelter and relevant parties can clarify emergency medical care expectations. The comfort and welfare of the animal is the shelter’s highest concern. The legal status of the animal must not prevent treatment to relieve suffering. This includes providing euthanasia if suffering cannot be alleviated.
6.5.3 Responding to infectious disease
Shelters must have a means of isolating contagious animals. Animals with a suspected infectious disease must be isolated until diagnosis by a veterinarian or treatment determines them to be a low risk to the general population. Isolation may be accomplished onsite or through placement in an appropriate facility, such as a veterinary clinic or foster home, after considering risk to animals already in those facilities. When isolation efforts are inadequate to prevent disease transmission to the population, informed adoption, transfer to a partner, or euthanasia of the infected animal needs to be considered. Allowing animals with severe infectious disease to remain in the general population is unacceptable.
The treatment and response plan for animals with mild to moderate or uncomplicated infections is based on circumstance and clinical signs and often follows a standard protocol. When the number of cases increases above typical for the shelter, when signs are severe or not responding to treatment as expected, and when a zoonotic condition is suspected, diagnosis or identification of specific pathogens should be sought. Individual animals, or a representative sample in an outbreak, can be tested to achieve a likely diagnosis. When an animal dies from unexplained causes, a necropsy should be performed.21 If gross necropsy is inconclusive, additional testing may be indicated.
6.5.4 Outbreak response
An outbreak is the occurrence of more than the usual number of animals affected by a disease or syndrome, or an increase in the severity of cases. Outbreaks can involve one animal or many animals; high levels of disease may represent an ongoing outbreak or gaps in management and preventive care practices.
During an outbreak, a risk assessment to identify potentially exposed animals must be performed based on the confirmed or suspected pathogen. Physical separation must be established between sick, exposed, at-risk, and unexposed animals or groups of animals. Implementation of this separation will vary depending on the disease of concern and type of facility. In some circumstances, isolation or limited handling of an animal or group of animals may be enough to protect the population. In other circumstances, it may be necessary to stop animal movement, including halting intake. In order to prevent tracking of pathogens from contaminated to uncontaminated areas, animal handling and foot traffic should be limited during disease outbreaks.
During an outbreak, all at-risk animals should be monitored for signs of disease at least once a day. Animal care staff should be educated on the clinical signs of the disease of concern and on the process for alerting medical staff. Shelters should avoid returning recovered or exposed animals to the general population, while there is significant risk that they may transmit disease to other animals. Shelters must also ensure federal, state, and local laws are followed concerning reportable diseases.
As part of the outbreak response, relevant protocols should be reviewed to ensure control measures are effective against the suspected pathogen. Effective measures, such as sanitation and animal handling protocols, help to ensure animal care and treatment activities do not contribute to the spread of disease. For example, footbaths often become contaminated and aid in disease transmission rather than control75 (see Sanitation).
Depopulation is defined as euthanasia of an entire population or subpopulation, including healthy and unhealthy animals. It is not an appropriate initial response to disease outbreaks and typically does not resolve the underlying causes. Depopulation is a technique of last resort reserved for extraordinary circumstances when morbidity, mortality, infectivity, injury, or risk of zoonotic disease is uncommonly severe. In the rare instance that depopulation is considered, an experienced shelter veterinarian must be consulted beforehand.76
6.6 Population health surveillance
Regular monitoring of population health is as important as monitoring individual animal health; one cannot exist without the other in the shelter environment. Shelters should track animal population health trends (e.g. morbidity and mortality) and develop targeted strategies to address concerns. Population health surveillance will facilitate early recognition of problems, accurate diagnoses, and effective intervention and prevention strategies.
One or more shelter animals dying in care can be a signal to assess management practices. Increases in deaths or infections over time may indicate deficiencies in population management practices, such as operating beyond a shelter’s capacity for care, lapses in preventive care protocols, or the need for targeted interventions. Shelters can learn from examples where conditions created by poor management caused severe suffering and unnecessary death.77,78
6.7 Rehoming considerations
It is increasingly common for shelters to find live outcomes for animals with medical conditions. Adopters or others receiving animals from shelters should be informed about any disease or condition known to be present at the time of outcome. Many shelters employ standard written disclosures for common conditions, modifying as needed for a particular animal.
Ongoing care for known medical conditions typically becomes the responsibility of the adopter, transport partner, or other caretaker of the animal, but may be provided by the shelter when regulations and policies allow. Shelters should have and disclose policies that specify whether or not they provide care for medical conditions that are ongoing or occur after adoption.
References
| 1. | World Health Organization. Constitution of the World Health Organization. American Journal of Public Health 36:11. 1946:1315–1323. |
| 2. | Ryan S, Bacon H, Endenburg N, et al. WSAVA Animal Welfare Guidelines. J Small Anim Pract. 2019;60(5):E1–E46. doi: 10.1111/JSAP.12998 |
| 3. | Ellis J, Marziani E, Aziz C, Brown CM, Cohn LA, Lea C, Moore GE, Taneja N. 2022 AAHA Canine Vaccination Guidelines. J Am Anim Hosp Assoc. 2022 Sep 1;58(5):213–230. doi: 10.5326/JAAHA-MS-Canine-Vaccination-Guidelines |
| 4. | Ford RB, Larson LJ, Mcclure KD, et al. 2017 AAHA Canine Vaccination Guidelines. 2017:26–35. Accessed Dec 13, 2022. https://www.aaha.org/public_documents/guidelines/vaccination_recommendation_for_general_practice_table.pdf. |
| 5. | American Association of Feline Practitioners. AAFP Position Statement: Welfare of Shelter Cats. 2009. Accessed Dec 13, 2022. https://catvets.com/guidelines/position-statements/welfare-shelter-cats |
| 6. | Larson LJ, Schultz RD. Canine and Feline Vaccinations and Immunology. In: Miller L, Janeczko S, Hurley KF, eds. Infectious Disease Management in Animal Shelters. 2nd ed. Hoboken, NJ: Wiley Blacklwell; 2021:191–220. |
| 7. | Spindel M. Strategies for Management of Infectious Disease in a Shelter. In: Miller L, Zawistowski SL, eds. Shelter Medicine for Veterinarians and Staff. 2nd ed. Ames, IA: Wiley Blackwell; 2013:281–286. |
| 8. | Scarlett JM, Greenberg MJ, Hoshizaki T. Every Nose Counts: Using Metrics in Animal Shelters. 1st ed. Ithaca, NY: CreateSpace Independent Publishing Platform; 2017. |
| 9. | Newbury S, Hurley K. Population Management. In: Miller L, Zawistowski S, eds. Shelter Medicine for Veterinarians and Staff. 2nd ed. Ames, IA: Wiley Blackwell; 2013:93–113. |
| 10. | American Veterinary Medical Association (AVMA). AVMA Policy: Model Veterinary Practice Act. J Am Vet Med Assoc. 2021. Accessed Dec 13, 2022. https://www.avma.org/sites/default/files/2021-01/model-veterinary-practice-act.pdf. Accessed January 12, 2022. |
| 11. | Griffin B. Wellness. In: Miller L, Janeczko S, Hurley KF, eds. Infectious Disease Management in Animal Shelters. 2nd ed. Hoboken, NJ: Wiley Blackwell; 2021:13–45. |
| 12. | Association of Shelter Veterinarians. Position Statement: Veterinary Supervision in Animal Shelters. 2021:1. Accessed Dec 13, 2022. https://www.sheltervet.org/assets/docs/position-statements/Veterinary Supervision in Animal Shelters PS 2021.pdf. |
| 13. | Fajt VR. Pharmacology. In: Miller L, Janeczko S, Hurley K, eds. Infectious Disease Management in Animal Shelters. 2nd ed. Hoboken, NJ: Wiley Blackwell; 2021:143–166. |
| 14. | American Veterinary Medical Association. Policy: Use of Prescription Drugs in Veterinary Medicine. 2022. Accessed Dec 13, 2022. https://www.avma.org/resources-tools/avma-policies/use-prescription-drugs-veterinary-medicine. |
| 15. | Federal Drug Administration. FDA Regulation of Animal Drugs. 2019. Accessed Dec 13, 2022. https://www.fda.gov/animal-veterinary/resources-you/fda-regulation-animal-drugs. |
| 16. | American Veterinary Medical Association. AVMA Policy: Companion Animal Care Guidelines. Accessed Dec 13, 2022. https://www.avma.org/policies/companion-animal-care-guidelines. |
| 17. | Hurley KF. The Evolving Role of Triage and Appointment-Based Admission to Improve Service, Care and Outcomes in Animal Shelters. Front Vet Sci. 2022;9:809340. doi: 10.3389/fvets.2022.809340 |
| 18. | UC Davis Koret Shelter Medicine Program. Performing a physical exam on a shelter animal. 2010. Accessed Dec 13, 2022. https://www.sheltermedicine.com/library/resources/?r=performing-a-physical-exam-on-a-shelter-animal. |
| 19. | Little S, Levy J, Hartmann K, et al. 2020 AAFP Feline Retrovirus Testing and Management Guidelines. J Feline Med Surg. 2020;22(1):5–30. doi: 10.1177/1098612X19895940 |
| 20. | Association of Shelter Veterinarians Position Statement: FeLV and FIV Testing and Management in Animal Shelters, 2020. Accessed Dec 13, 2022. https://www.sheltervet.org/assets/docs/position-statements/Retroviral%20PS.pdf. |
| 21. | Jenkins E, Davis C, Carrai M, et al. Feline Parvovirus Seroprevalence Is High in Domestic Cats from Disease Outbreak and Non-Outbreak Regions in Australia. Viruses. 2020;12(3): 1–12. doi: 10.3390/v12030320 |
| 22. | Beatty JA, Hartmann K. Advances in Feline Viruses and Viral Diseases. Viruses. 2021;13(5):2–6. doi: 10.3390/v13050923 |
| 23. | Spindel M, Sykes JE. 16: Prevention and Management of Infectious Diseases in Multiple-Cat Environments. In: Sykes JE, ed. Greene’s Infectious Diseases of the Dog and Cat. 5th ed. Amsterdam: Elsevier; 2022:187–186. |
| 24. | Van Brussel K, Carrai M, Lin C, et al. Distinct Lineages of Feline Parvovirus Associated with Epizootic Outbreaks in Australia, New Zealand and the United Arab Emirates. Viruses. 2019;11(12):1–20. doi: 10.3390/v11121155 |
| 25. | Day MJ, Horzinek MC, Schultz RD, Squires RA. WSAVA Guidelines for the Vaccination of Dogs and Cats. J Small Anim Pract. 2016;57(1):E1–E45. doi: 10.1111/jsap.2_12431 |
| 26. | DiGangi BA. Strategies for Infectious Disease Management in Shelter Cats. In: Little S, ed. August’s Consultations in Feline Internal Medicine. Vol 7. First. St Louis, MO: Elsevier Inc.; 2016:674-685. doi: 10.1016/B978-0-323-22652-3.00070-0 |
| 27. | Davis-Wurzler GM. Current Vaccination Strategies in Puppies and Kittens. Vet Clin North Am Small Anim Pract. 2006;36(3):607–640. doi: 10.1016/j.cvsm.2005.12.003 |
| 28. | Stone A, Brummet GO, Carozza EM, et al. 2020 AAHA / AAFP Feline Vaccination Guidelines. J Feline Med Surg. 2020;22:813–830. doi: 10.1177/1098612X20941784 |
| 29. | Paul MA, Carmichael L, Childers H, et al. 2006 American Animal Hospital Association (AAHA) Canine Vaccine Guidelines. American Animal Hospital Association; 2006:80–89. |
| 30. | UC Davis Koret Shelter Medicine Program. Vaccination in Animal Shelters. Inf Sheet Infect Dis. 2015. Accessed Dec 13, 2022. https://www.sheltermedicine.com/library/resources/?r=vaccination-in-animal-shelters. |
| 31. | Gershwin LJ. Adverse Reactions to Vaccination: From Anaphylaxis to Autoimmunity. Vet Clin North Am Small Anim Pr. 2018;48(2):279–290. doi: 10.1016/j.cvsm.2017.10.005 |
| 32. | United States Department of Agriculture Animal and Plant Health Inspection Service. Adverse Event Reporting. 2022. Accessed Dec 13, 2022. https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/veterinary-biologics/adverse-event-reporting/ct_vb_adverse_event. |
| 33. | Larson LJ, Schultz RD. Effect of Vaccination with Recombinant Canine Distempber Virus Vaccine Immediately before Exposure under Shelter-Like Conditions. Vet Ther. 2006;7(2):113–118. |
| 34. | Lappin MR. Feline Panleukopenia Virus, Feline Herpesvirus-1 and Feline Calicivirus Antibody Responses in Seronegative Specific Pathogen-Free Kittens after Parenteral Administration of an Inactivated FVRCP Vaccine or a Modified Live FVRCP Vaccine. J Feline Med Surg. 2012;14(2):161–164. doi: 10.1177/1098612X11432240 |
| 35. | Digangi BA, Levy JK, Griffin B, et al. Effects of Maternally-Derived Antibodies on Serologic Responses to Vaccination in Kittens. J Feline Med Surg. 2012;14(2):118–123. doi: 10.1177/1098612X11432239 |
| 36. | Fischer S, Quest C, Dubovi E, et al. Response of Feral Cats to Vaccination at the Time of Neutering. J Am Vet Med Assoc. 2007;230(1):52–58. doi: 10.2460/javma.230.1.52 |
| 37. | Barrs VRV. Feline Panleukopenia: A Re-Emergent Disease. Vet Clin North Am Small Anim Pract. 2019;49(4):651–670. doi: 10.1016/j.cvsm.2019.02.006 |
| 38. | De Medeiros Oliveira IVP, De Carvalho Freire DA, Ferreira HIP, et al. Research on Viral Agents Associated with Feline Reproductive Problems Reveals a High Association with Feline Panleukopenia Virus. Vet Anim Sci. 2018;6:75–80. doi: 10.1016/j.vas.2018.06.004 |
| 39. | Lappin MR, Veir J, Hawley J. Feline Panleukopenia Virus, Feline Herpesvirus-1, and Feline Calicivirus Antibody Responses in Seronegative Specific Pathogen-Free Cats after a Single Administration of Two Different Modified Live FVRCP Vaccines. J Feline Med Surg. 2009;11(2):159–162. doi: 10.1016/j.jfms.2008.05.004 |
| 40. | Jas D, Aeberlé C, Lacombe V, Guiot AL, Poulet H. Onset of Immunity in Kittens after Vaccination with a Non-Adjuvanted Vaccine against Feline Panleucopenia, Feline Calicivirus and Feline Herpesvirus. Vet J. 2009;182(1):86–93. doi: 10.1016/j.tvjl.2008.05.025 |
| 41. | Cunha RDS, Da Silva Junior CL, Costa CA, De Aguiar HM, Junqueira Júnior DG. Comparison of Immunity against Canine Distemper, Adenovirus and Parvovirus after Vaccination with Two Multivalent Canine Vaccines. Vet Med Sci. 2020;6(3):330–334. doi: 10.1002/vms3.274 |
| 42. | Bergmann M, Schwertler S, Speck S, Truyen U, Hartmann K, Bergman M. Antibody Response to Feline Panleukopenia Virus Vaccination in Cats with Asymptomatic Retrovirus Infections: A Pilot Study. J Feline Med Surg. 2019;21(12):1094–1101. doi: 10.1177/1098612X18816463 |
| 43. | Ellis JA, Gow SP, Waldner CL, et al. Comparative Efficacy of Intranasal and Oral Vaccines against Bordetella Bronchiseptica in Dogs. Vet J. 2016;212:71–77. doi: 10.1016/j.tvjl.2016.04.004 |
| 44. | Ellis JA, Gow SP, Lee LB, Lacoste S, Ball EC. Comparative Efficacy of Intranasal and Injectable Vaccines in Stimulating Bordetella Bronchiseptica-Reacììve Anamnestic Antibody Responses in Household Dogs. Can Vet J. 2017;58(8):809–815. |
| 45. | Brown CM, Slavinski S, Ettestad P, Sidwa TJ, Sorhage FE. Compendium of Animal Rabies Prevention and Control, 2016. J Am Vet Med Assoc. 2016;248(5):505–517. doi: 10.2460/javma.248.5.505 |
| 46. | American Veterinary Medical Association: Government Relations. State Rabies Vaccinations Laws. 2021:13. Accessed Dec 13, 2022. https://www.avma.org/sites/default/files/2021-08/State-Rabies-Vaccination-Laws-Chart.pdf. |
| 47. | Moore MC, Davis RD, Kang Q, et al. Comparison of Anamnestic Responses to Rabies Vaccination in Dogs and Cats with Current and Out-of-Date Vaccination Status. J Am Vet Med Assoc. 2015;246:205–211. doi: 10.2460/javma.246.2.205 |
| 48. | Smith K, Dunn J, Castrodale L, Wohrle R. Compendium of Measures to Prevent Disease Associated with Animals in Public Settings, 2013. Javma. 2016;248(5):1997–2001. doi: 10.2460/javma.248.5.505 |
| 49. | Levy JK, Wilford CL. Management of Stray and Feral Community Cats. In: Miller L, Zawistowski SL, eds. Shelter Medicine for Veterinarians and Staff. 2nd ed. Ames, IA: Wiley-Blackwell; 2013:669–688. |
| 50. | Jacobson LS. 18: Considerations and Management of Infectious Diseases of Community (Unowned, Free-Roaming) Cats. In: Sykes JE, ed. Greene’s Infectious Diseases of the Dog and Cat. 5th ed. Amsterdam: Elsevier; 2022:204–218. |
| 51. | Griffin B, Bushby PA, Mccobb E, et al. The Association of Shelter Veterinarians’ 2016 Veterinary Medical Care Guidelines for Spay-Neuter Programs. J Am Vet Med Assoc. 2016;249(2):165–188. doi: 10.2460/javma.249.2.165 |
| 52. | Miyamoto T, Taura Y, Une S, Yoshitake M, Nakama S, Watanabe S. Immunological Resonses after Vaccination Pre- and Post-Surgery in Dogs. J Vet Med Sci. 1995;57(1):29–32. doi: 10.1292/jvms.57.29 |
| 53. | Reese MJ, Patterson EV, Tucker SJ, et al. Effects of Anesthesia and Surgery on Serologic Responses to Vaccination in Kittens. J Am Vet Med Assoc. 2008;233(1):116–121. doi: 10.2460/javma.233.1.116 |
| 54. | Miller L, Janeczko S. Canine Care in the Animal Shelter. In: Miller L, Zawistowski SL, eds. Shelter Medicine for Veterinarians and Staff. 2nd ed. Ames, IA: Wiley Blackwell; 2013:115–144. doi: 10.1002/9781119421511.ch9 |
| 55. | Vila Nova B, Cunha E, Sepúlveda N, et al. Evaluation of the Humoral Immune Response Induced by Vaccination for Canine Distemper and Parvovirus: A Pilot Study. BMC Vet Res. 2018;14(1):1–8. doi: 10.1186/s12917-018-1673-z |
| 56. | Raza A, Rand J, Qamar AG, Jabbar A, Kopp S. Gastrointestinal Parasites in Shelter Dogs: Occurrence, Pathology, Treatment and Risk to Shelter Workers. Animals. 2018;8(7):1–23. doi: 10.3390/ani8070108 |
| 57. | Levy JK, Lappin MR, Glaser AL, Birkenheuer AJ, Anderson TC, Edinboro CH. Prevalence of Infectious Diseases in Cats and Dogs Rescued Following Hurricane Katrina. J Am Vet Med Assoc. 2011;238(3):311–317. doi: 10.2460/javma.238.3.311 |
| 58. | Loftin CM, Donnett UB, Schneider LG, Varela-Stokes AS. Prevalence of Endoparasites in Northern Mississippi Shelter Cats. Vet Parasitol Reg Stud Reports. 2019;18:100322. doi: 10.1016/j.vprsr.2019.100322 |
| 59. | Nagamori Y, Payton ME, Duncan-Decocq R, Johnson EM. Fecal Survey of Parasites in Free-Roaming Cats in Northcentral Oklahoma, United States. Vet Parasitol Reg Stud Reports. 2018;14:50–53. doi: 10.1016/j.vprsr.2018.08.008 |
| 60. | Nagamori Y, Payton ME, Looper E, Apple H, Johnson EM. Retrospective Survey of Parasitism Identified in Feces of Client-Owned Cats in North America from 2007 through 2018. Vet Parasitol. 2020;277:109008. doi: 10.1016/j.vetpar.2019.109008 |
| 61. | Companion Animal Parasite Council. CAPC Quick Product Reference Guide. Accessed Dec 13, 2022. https://capcvet.org/parasite-product-applications/ |
| 62. | Boyce J, Pittet D. Morbidity and Mortality Weekly Report Guideline for Hand Hygiene in Health-Care Settings Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Centers for Disease Control and Prevention; 2002;51. Accessed Dec 13, 2022. https://www.cdc.gov/mmwr/PDF/rr/rr5116.pdf. |
| 63. | Committee NA of SPHVVIC. Compendium of Veterinary Standard Precautions for Zoonotic Disease Prevention in Veterinary Personnel. JAVMA. 2015;247(11):1252–1265. doi: 10.2460/javma.247.11.1252 |
| 64. | Smith M, American Humane. Operational Guide: Sanitation and Disease Control in the Shelter Environment. 2010. Accessed Dec 13, 2022. http://unddr.org/uploads/documents/OperationalGuide.pdf. |
| 65. | Association of Shelter Veterinarians. Heartworm Management. 2018. Accessed Dec 13, 2022. https://www.sheltervet.org/assets/docs/position-statements/Heartworm. |
| 66. | Polak KC, Smith-Blackmore M. Animal Shelters: Managing Heartworms in Resource-Scarce Environments. Vet Parasitol. 2014;206(1–2):78–82. doi: 10.1016/j.vetpar.2014.03.023 |
| 67. | Drake J, Parrish RS. Dog Importation and Changes in Heartworm Prevalence in Colorado 2013–2017. Parasit Vectors. 2019;12(1):207. doi: 10.1186/s13071-019-3473-0 |
| 68. | American Heartworm Society, Association of Shelter Veterinarians. Minimizing Heartworm Transmission in Relocated Dogs. 2017. Accessed Dec 13, 2022. https://www.sheltervet.org/assets/PDFs/Relocating%20HW%2BDogs.pdf |
| 69. | Jacobson LS, DiGangi BA. An Accessible Alternative to Melarsomine: ‘Moxi-Doxy’ for Treatment of Adult Heartworm Infection in Dogs. Front Vet Sci. 2021;8:1–17. doi: 10.3389/fvets.2021.702018 |
| 70. | Griffin B. Feline Care in the Animal Shelter. In: Miller L, Zawistowski SL, eds. Shelter Medicine for Veterinarians and Staff. 2nd ed. Ames, IA: Wiley-Blackwell; 2013:145–184. doi: 10.1002/9781119421511.ch10 |
| 71. | Smith FO. Prenatal Care of the Bitch and Queen. Small Anim Pediatr. 2011;1:1–10. doi: 10.1016/B978-1-4160-4889-3.00001-2 |
| 72. | Robertson SA. What Is Pain? J Am Vet Med Assoc. 2002;221:202–205. doi: 10.1016/S0140-6736(02)39134-7 |
| 73. | Paul-Murphy J, Ludders JW, Robertson SA, Gaynor JS, Hellyer PW, Wong PL. The Need for a Cross-Species, Approach to the Study of Pain in Animals. J Am Vet Med Assoc. 2004;224(5): 692–697. doi: 10.2460/javma.2004.224.692 |
| 74. | Epstein M, Rodan I, Griffenhagen G, et al. 2015 AAHA/AAFP Pain Management Guidelines for Dogs and Cats. J Am Anim Hosp Assoc. 2015;51(2):67–84. doi: 10.5326/JAAHA-MS-7331 |
| 75. | Amass SF, Abvp D, Vlwerberg BD, Ragland D, Dowell CA, Anderson CD. Evaluating the Efficacy of Boot Baths in Biosecurier Protocols. Swine Heal Prod. 2000;8(4):169–173. |
| 76. | Association of Shelter Veterinarians. Position Statement: Depopulation. 2020. Accessed April 5, 2020. https://www.sheltervet.org/assets/docs/position-statements/Depopulation PS 3.20.pdf. |
| 77. | James L. 14 Animal Deaths at Pueblo Shelter Lead to State Takeover. Gazette. 2019. Accessed Dec 13, 2022. https://gazette.com/news/14-animal-deaths-at-pueblo-shelter-lead-to-state-takeover/article_f1201cce-50a4-11e9-84a4-67ccc1f98fed.html. |
| 78. | The HSUS Animal Services Consultation Program. The Animal Foundation Lied Animal Shelter, Las Vegas NV. Washington, DC: Humane Society of the United States; 2007. |
7. Shelter Surgery
7.1 General
In order to decrease the local population of animals needing shelter services and improve individual animal health and welfare, shelters routinely sterilize (i.e. spay or neuter) shelter animals, owned pets, and community cats. Robust community spay-neuter programs target pets and free-roaming cats who might not otherwise have been sterilized. This, in turn, supports community animal health, prevents shelter intake, and reduces euthanasia of both adults and unplanned offspring.1–6 Spay-neuter is associated with a reduction in many nuisance and unwanted behaviors7–9 and is associated with increased life expectancy.10,11 In some jurisdictions, pre-adoption sterilization of dogs and cats is required by law.
Many areas of the country continue to deal with pet overpopulation, and it is important for shelters not to exacerbate this problem.12 The severity of overpopulation varies on local, regional, and national levels as well as by species. It is unacceptable for organizations to allow shelter animals to breed. When spay-neuter is not immediately available, housing intact animals of breeding age separately or in sex-matched pairs and thoughtfully planning and monitoring off-leash activities such as playgroups can prevent mating behaviors.
When animals that are already pregnant are admitted, shelters should prevent birth from occurring in the facility, instead seeking alternatives such as spay or foster care. In almost all cases, it is safe and humane to spay dogs and cats at any stage of pregnancy. Keeping the uterus closed during and following the spay procedure allows the anesthetized fetuses to die humanely without the need for additional barbiturate injections.13 If a shelter is considering allowing animals to give birth, it is important to assess the availability of routine and after-hours emergency medical care, behavioral care, foster home capacity, live outcome options, and regional population implications.
7.2 Spay-Neuter
Shelters should sterilize all animals before adoption or ensure that they will be sterilized after their outcome. Performing spay-neuter prior to adoption ensures completion and reduces the risk of additional litters prior to surgery. Spay-neuter can be safely performed in healthy animals as young as 6 weeks old and as small as 1.5–2 pounds (0.7–1 kg) body weight.14–17 If a shelter does not have the capacity to sterilize all animals prior to adoption without increasing length of stay, an acceptable alternative is to arrange post-adoption spay-neuter. Shelters performing post-adoption sterilization must have a system for keeping track of unaltered animals and ensuring that surgery is completed in a timely manner. As adopters may be unfamiliar with the needs and care of sexually intact animals, providing information about the reproductive cycle, potential medical and behavior issues, and preventing breeding is recommended.
In some situations, spay-neuter surgery or the anesthesia it requires puts an animal’s health at risk.18 The final decision regarding acceptance of any patient for surgery must be made by a veterinarian based on a physical examination, available medical history, and capacity of the surgical team. Granting an exemption from a spay-neuter requirement should only occur when performing the procedure puts the patient at significant risk. It is generally safe to sterilize patients in estrus or suffering from mild infections or other medical conditions, such as infectious respiratory disease or heartworm disease.19,20 When considering sterilizing patients with medical conditions, veterinarians must weigh the benefits and risks to that animal, others receiving surgery that day, the shelter population, and the community population. Shelter spay-neuter policies need to follow all state and local ordinances regarding the timing of spay-neuter with respect to legal holding periods.
7.2.1 Practices and protocols
Shelters that perform their own sterilization surgeries must follow the current ASV Veterinary Medical Care Guidelines for Spay-Neuter Programs, which includes establishing policies and protocols for managing related complications and emergencies.19 This document provides guidance on presurgical care, transport, anesthesia, pain management, surgery, and postsurgical care. It is also recommended that outside veterinary partners who work with shelters be familiar with the ASV Spay-Neuter Guidelines. Shelters can refer to this document when discussing expectations for surgical care, pain control, and the management of postoperative complications with new surgeons and partners.
7.2.2 Identifying altered animals
Sterilization status should be documented for each animal. Spay scars can be difficult to see, and other surgeries or injuries can leave similar scars. The placement of a permanent tattoo on the abdomen at the time of spay-neuter is an accepted standard for indicating sterilization and strongly recommended for all animals.19,21 If an animal is lost or transferred to another owner without records, the tattoo can prevent unnecessary anesthesia or surgery. For community cats, removal of the tip of one ear is the accepted standard for indicating an animal is sterilized.19,21,22 The ears are visible from a distance without the need for handling, which helps with colony monitoring and prevents unnecessary transport of already sterilized cats.
7.3 Other surgeries
Some animals presenting to shelters have medical concerns that require surgical treatment. In shelters that regularly perform spay-neuter surgery, these non-sterilization surgical procedures may also be performed onsite. To promote quality care for surgical patients, all surgical practices and protocols must be developed in consultation with a veterinarian familiar with the sheltering organization, its population, and facilities.
Non-sterilization surgeries performed in the shelter setting, including dentistry, must adhere to the ASV Spay-Neuter Guidelines regarding surgical suite, anesthesia, analgesia, and principles of sterility related to instrumentation and surgical practice.19 Ideally, shelters without the capacity to perform these surgeries partner with outside organizations, specialists, or transport partners to obtain necessary care.
Regardless of where surgery is performed, it is critical that shelters pursue surgical treatment only when the appropriate pre- and postsurgical care can be provided. In particular, following orthopedic procedures, patients must receive appropriate rehabilitation and pain management in order to minimize discomfort and ensure success of the procedure.23 Due to often-prolonged recovery times for orthopedic patients and their special mobility and care needs, appropriate postoperative plans may require alternative housing plans such as foster care or adoption with in-depth counseling. Ideally, orthopedic patients requiring extended care are not housed long term at the shelter.
7.3.1 Dentistry
Providing surgical dental services is an increasingly common part of shelter animal care, particularly for geriatric animals.24–26 Appropriate dental care considers individual patient health, surgical safety, and postoperative recovery needs including pain control, in the context of the shelter population.27 Medical records should document the dental exam, diagnostics, and treatments performed.
Non-anesthetic dental probing, scaling, and polishing is unacceptable.28,29 Without sedation, significant dental concerns can be missed or inadequately addressed. The restraint required can cause significant animal and technician stress, and veterinary staff and the animal are put at risk of serious injury from sharp instruments or bites.28,29
Ideally, intraoral radiographs are taken in patients undergoing dental surgery. Radiographs allow veterinarians to detect important concerns of the tooth and jaw not visible during oral examination.28,29 Dental disease can have serious welfare implications, and treatment for a painful mouth is strongly recommended even when radiology is not available. Dental procedures, including radiology, must be performed by appropriately trained and credentialed individuals based on state and local regulations.28 Shelters without the capacity to perform dentistry can partner with adopters, outside organizations, specialists, or transport partners to ensure animals receive needed care.
References
| 1. | Levy JK, Isaza NM, Scott KC. Effect of High-Impact Targeted Trap-Neuter-Return and Adoption of Community Cats on Cat Intake to a Shelter. Vet J. 2014;201(3):269–274. doi: 10.1016/j.tvjl.2014.05.001 |
| 2. | Spehar DD, Wolf PJ. The Impact of an Integrated Program of Return-to-Field and Targeted Trap-Neuter-Return on Feline Intake and Euthanasia at a Municipal Animal Shelter. Animals. 2018;8(4):55. doi: 10.3390/ani8040055 |
| 3. | Spehar DD, Wolf PJ. The Impact of Return-to-Field and Targeted Trap-Neuter-Return on Feline Intake and Euthanasia at a Municipal Animal Shelter in Jefferson County, Kentucky. Animals. 2020;10(8):1–18. doi: 10.3390/ani10081395 |
| 4. | Spehar DD, Wolf PJ. The Impact of Targeted Trap–Neuter–Return Efforts in the San Francisco Bay Area. Animals. 2020;10(11):1–12. doi: 10.3390/ani10112089 |
| 5. | Scarlett J, Johnston N. Impact of a Subsidized Spay Neuter Clinic on Impoundments and Euthanasia in a Community Shelter and On Service and Complaint Calls to Animal Control. J Appl Anim Welf Sci. 2012;15(1):53–69. doi: 10.1080/10888705.2012.624902 |
| 6. | White SC, Jefferson E, Levy JK. Impact of Publicly Sponsored Neutering Programs on Animal Population Dynamics at Animal Shelters: The New Hampshire and Austin Experiences. J Appl Anim Welf Sci. 2010;13(3):191–212. doi: 10.1080/10888700903579903 |
| 7. | Patronek GJ, Glickman LT, Beck A, McCabe G, Ecker C. Risk Factors for Relinquishment of Dogs to an Animal Shelter. J Am Vet Med Assoc. 1996;209(3):572–581. |
| 8. | Patronek GJ, Glickman LT, Beck A, McCabe G, Ecker C. Risk Factors for Relinquishment of Cats to an Animal Shelter. J Am Vet Med Assoc. 1996;209(3):582–588. |
| 9. | Dolan ED, Scotto J, Slater M, Weiss E. Risk Factors for Dog Relinquishment to a Los Angeles Municipal Animal Shelter. Anim. 2015;5(4):1311–1328. doi: 10.3390/ani5040413 |
| 10. | Hoffman JM, Creevy KE, Promislow DEL. Reproductive Capability Is Associated with Lifespan and Cause of Death in Companion Dogs. PLoS One. 2013;8(4):e61082. doi: 10.1371/journal.pone.0061082 |
| 11. | Banfield Pet Hospital. State of Pet Health 2013 Report. 2013. Accessed Dec 13, 2022. https://www.banfield.com/-/media/Project/Banfield/Main/en/general/SOPH-Infographic/PDFs/Banfield-State-of-Pet-Health-Report_2013.pdf?rev=a8612f3fa39141e3bf2876a5ed6760de&hash=D79B771D2C3539DF737353E65D310504 |
| 12. | Weedon GR, Root Kustritz MV, Bushby PA. Influence of Spay-Neuter Timing on Health. In: White S, ed. High-Quality High-Volume Spay and Neuter and Other Shelter Surgeries. 1st ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2019:509–520. |
| 13. | White SC. Prevention of Fetal Suffering during Ovariohysterectomy of Pregnant Animals. J Am Vet Med Assoc. 2012;240(10):1160–1163. doi: 10.2460/javma.240.10.1160 |
| 14. | Root Kustritz MV. Determining the Optimal Age for Gonadectomy of Dogs and Cats. J Am Vet Med Assoc. 2007;231(11):1665–1675. doi: 10.2460/javma.231.11.1665 |
| 15. | Spain CV, Scarlett JM, Houpt KA. Long-Term Risks and Benefits of Early-Age Gonadectomy in Cats. J Am Vet Med Assoc. 2004;224(3):372–379. doi: 10.2460/javma.2004.224.372 |
| 16. | Howe LM, Slater MR, Boothe HW, Hobson HP, Holcom JL, Spann AC. Long-Term Outcome of Gonadectomy Performed at an Early Age or Traditional Age in Dogs. J Am Vet Med Assoc. 2001;218(2):217–221. doi: 10.2460/javma.2001.218.217 |
| 17. | Howe LM, Slater MR, Boothe HW, Hobson HP, Holcom JL, Spann AC. Long-Term Outcome of Gonadectomy Performed at an Early Age or Traditional Age in Cats. J Am Vet Med Assoc. 2000;217(11):1661–1665. doi: 10.2460/javma.2001.218.217 |
| 18. | Robertson S. Principles of Anesthesia, Analgesia, Safety, and Monitoring. In: White S, ed. High-Quality High-Volume Spay and Neuter and Other Shelter Surgeries. 1st ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2020:125–152. |
| 19. | Griffin B, Bushby PA, Mccobb E, et al. The Association of Shelter Veterinarians’ 2016 Veterinary Medical Care Guidelines for Spay-Neuter Programs. J Am Vet Med Assoc. 2016;249(2):165–188. |
| 20. | Peterson KM, Chappell DE, Lewis B, et al. Heartworm-Positive Dogs Recover without Complications from Surgical Sterilization Using Cardiovascular Sparing Anesthesia Protocol. Vet Parasitol. 2014;206(1–2):83–85. doi: 10.1016/j.vetpar.2014.08.017 |
| 21. | Griffin B. Determination of Patient Sex and Spay-Neuter Status. In: White S, ed. High-Quality High-Volume Spay and Neuter and Other Shelter Surgeries. 1st ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2020:1–25. |
| 22. | Dalrymple AM, MacDonald LJ, Kreisler RE. Ear-Tipping Practices for Identification of Cats Sterilized in Trap–Neuter–Return Programs in the USA. J Feline Med Surg. 2022. doi: 10.1177/1098612X221105843 |
| 23. | Epstein M, Rodan I, Griffenhagen G, et al. 2015 AAHA/AAFP Pain Management Guidelines for Dogs and Cats. J Am Anim Hosp Assoc. 2015;51(2):67–84. doi: 10.5326/JAAHA-MS-7331 |
| 24. | Whyte A, Gracia A, Bonastre C, et al. Oral Disease and Microbiota in Free-Roaming Cats. Top Companion Anim Med. 2017;32(3):91–95. doi: 10.1053/j.tcam.2017.07.003 |
| 25. | Janse JM. Medical Differences between Stray and Owner Surrendered Dogs in Dutch Animal Shelters. 2014. University of Utrecht, Netherlands. |
| 26. | Steneroden KK, Hill AE, Salman MD. A Needs-Assessment and Demographic Survey of Infection-Control and Disease Awareness in Western US Animal Shelters. Prev Vet Med. 2011;98(1):52–57. doi: 10.1016/j.prevetmed.2010.11.001 |
| 27. | Eubanks DL, Love L. Dental Extractions in a Shelter Environment. In: White S, ed. High-Quality, High-Volume Spay and Neuter and Other Shelter Surgeries. 1st ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2019:425–436. |
| 28. | Bellows J, Berg ML, Dennis S, et al. 2019 AAHA Dental Care Guidelines for Dogs and Cats. J Am Anim Hosp Assoc. 2019;55(2):49–69. doi: 10.5326/JAAHA-MS-6933 |
| 29. | Niemiec B, Gawor J, Nemec A, et al. World Small Animal Veterinary Association Global Dental Guidelines. J Small Anim Pract. 2020;61:1–151. |
8. Forensics
8.1 General
All animal shelters play an important role in the prevention of animal suffering. Socioeconomic factors often place owners in situations with limited access to veterinary care or difficulty meeting their pet’s basic care needs.1 This can lead owners to surrender their pets or result in seizure if a complaint is filed. In many cases, shelters can help owners and their pets by providing necessary services (e.g. food, medical care, shelter, and grooming) and information, or connecting owners with others in the community who can assist them.
While community interventions are an important strategy to improve animal welfare, any shelter may receive animals who have experienced abuse or neglect (i.e. maltreatment). Shelters have an obligation to recognize and report suspected cases. Many shelters are engaged in the active investigation of suspected crimes against animals, or forensics, which can be part of a their mission or mandate.2 Caring for animals who have been abused or neglected may place significant demands on shelter resources due to their medical or behavioral needs, the number of animals involved, and potentially lengthy stays while a legal outcome is determined.
8.2 Laws and regulations
The definitions of animal abuse and neglect vary across states and jurisdictions, as do relevant laws.3,4 These crimes range from inflicting physical or emotional harm (i.e. abuse) to failing to provide adequate and necessary care (i.e. neglect).5–7 Shelters, veterinarians, and humane investigators must be familiar with animal abuse and neglect laws in their jurisdiction and know how to report suspected cases. In recent years, the Five Domains model of animal welfare assessment has been used as a framework for assessment in animal legal cases.8,9
In several states, veterinarians have been designated as mandated reporters of animal abuse and neglect. Most of these states provide protection from liability (i.e. law suits) for those who report suspected crimes in good faith; however, reporting is important regardless.2,4,10 Veterinarians must be aware of their state’s animal cruelty reporting requirements and liability protection statutes. In some states, veterinarians and other shelter personnel may also be required to report suspected abuse and neglect of people.
8.3 Forensic investigation policies
Shelters should have a policy that outlines the scope of forensic services provided. Services may be limited to animal care or may involve active investigation. For shelters that regularly perform investigations or provide investigative support to other agencies, the forensic investigation policy needs to define:
- which geographic areas are covered
- which species can be investigated
- where forensic exams are performed
- who performs forensic exams
- how animals and other evidence are held10,11
Consultation with an attorney is suggested during the development of a forensic investigation policy.2
Sharing the shelter’s forensic investigations policy helps partner agencies understand how and when the shelter may be able to assist. A memorandum of understanding (MOU) with collaborating agencies defines roles and financial responsibilities for crime scene documentation, the care and treatment of animals, and allows an orderly investigation response. When law enforcement agencies are leading an investigation, a release permitting the shelter to examine and care for the animals is recommended.5,6,11,12
Those investigating a suspected case of animal abuse or neglect must first ensure that they have the legal right (e.g. seizure, warrant, or owner consent) to examine, treat, and document the condition of the animal or scene.10 It is essential that all involved in the investigation of animal abuse and neglect understand the legal procedures involved in criminal investigation, including the defendant’s right to protection from unreasonable search and seizure. Mishandling evidence can cause it to be withheld from court proceedings.3,5,7,12–14
8.4 The veterinary forensic evaluation
Veterinary forensic evaluations are holistic assessments of all aspects of an animal abuse or neglect case. The veterinarian should have access to information about the scene, evidence collected, allegations, and known or reported history.15,16 The veterinary forensic evaluation includes all of this information, as well as findings from forensic examination or necropsy, diagnostic results, and evidence collected from the animal.5,11,14 Evaluation and opinion formation for forensic purposes must be conducted by a veterinarian.
Veterinarians involved in forensic cases may be expected to provide evidence through written statements or by providing testimony in court.17,18 The lead investigator or district attorney is a good resource for understanding legal expectations and requirements.5,14,17 The goal of the veterinarian’s report and testimony is to present and interpret the facts of the case. It is up to the prosecution to prove the case, and the jury or judge to decide.7,18
8.4.1 Veterinary forensic examination
A key part of forensic evaluation is a forensic physical exam or necropsy with documentation, for which shelters should have standard protocols.19–21 These protocols ensure that each forensic examination is approached consistently and methodically. Further diagnostics, treatments, or assessments can be performed based on presentation and initial findings.22–25
When animals have urgent medical needs, the priority is providing stabilization and medical care. In most cases, this can be accomplished while simultaneously trying to identify, document, collect, and preserve key evidence. Even when cases are not medically urgent, forensic physical examinations and diagnostics must be conducted in a timely manner to preserve evidence. Case evidence may disappear quickly or change over time with appropriate care. For example, blood chemistry values may normalize after feeding and hydration, and trace evidence visible on the body under normal or alternate light sources may be lost during movement and grooming.22,26–32
8.4.2 Documentation
Photographs are essential when documenting evidence of suspected abuse and neglect. Standard views include the front, back, left, right, and top of the animal, as well as photos of abnormalities. At least one photo should include identifying information. Photographs should be of sufficient quality to serve as evidence, and they should be managed to ensure proof of origin and integrity.2,22,26,34 Videos can help document dynamic processes such as limping or behavior.19
8.5 Managing evidence
Humane investigators and veterinarians involved in investigating animal abuse and neglect must be prepared to maintain chain of custody protocols. To ensure proper packaging, storage, and transfer of evidence between agencies, it is recommended that shelters consult local law enforcement, the forensic laboratory, or forensics reference materials.12,13,29
Monitoring and response to ongoing treatment should be documented as evidence throughout recovery. Demonstrating improvement as a response to appropriate care provides evidence and may refute narratives presented by the defense.11,22,34 For example, a log of increasing weights accompanied by photographs of an animal recovering from emaciation contradicts an assertion that the animal was losing weight despite being given an adequate diet.
8.6 Training
Specific training regarding forensic evaluations, evidence identification and collection, testifying in court, and other aspects of forensic investigations has become widely accessible (Appendix D). Veterinarians routinely involved in the investigation of animal cruelty should complete additional training in veterinary forensics or criminal justice. Attending trainings for law enforcement or human medical professionals, including forensic nursing and medical examiners, can also be helpful.14
References
| 1. | Neal SM, Greenberg MJ. Veterinary Care Deserts: What Is the Capacity and Where Is It? J Shelter Med Community Heal. 2022;1(1):1–8. doi: 10.56771/jsmcah.v1.2 |
| 2. | Wolf S. Overview of Animal Cruelty Laws. In: Miller L, Zawistowski S, eds. Shelter Medicine for Veterinarians and Staff. 2nd ed. Ames, IA: Wiley Blackwell; 2013:369–382. |
| 3. | Welch M. Animal Law. In: Byrd JH, Norris P, Bradley-Siemens N, eds. Veterinary Forensic Medicine and Forensic Science. 1st ed. Boca Raton, FL: CRC Press; 2020:435–460. |
| 4. | Lockwood R, Arkow P. Animal Abuse and Interpersonal Violence. Vet Pathol. 2016;53(5):910–918. doi: 10.1177/0300985815626575 |
| 5. | Underkoffler S, Sylvia S. Humane Law Enforcement. In: Byrd JH, Norris P, Bradley-Siemens N, eds. Veterinary Forensic Medicine and Forensic Science. 1st ed. Boca Raton, FL: CRC Press; 2020:35–56. |
| 6. | Balkin D, Janssen L, Merck M. The Legal System: The Veterinarian’s Role and Responsibilities. In: Merck MD, ed. Veterinary Forensics: Animal Cruelty Investigations. 2nd ed. West Sussex: John Wiley & Sons, Inc.; 2012:1–16. doi: 10.1002/9781118704738 |
| 7. | Barr J-H. The Judicial System. In: Rogers ER, Stern AW, eds. Veterinary Forensics. 1st ed. Boca Raton, FL: CRC Press; 2018:381–388. |
| 8. | Ledger RA, Mellor DJ. Forensic Use of the Five Domains Model for Assessing Suffering in Cases of Animal Cruelty. Animals. 2018;8(7):1–19. doi: 10.3390/ani8070101 |
| 9. | Mellor DJ, Beausoleil NJ, Littlewood KE, et al. The 2020 Five Domains Model: Including Human–Animal Interactions in Assessments of Animal Welfare. Anim. 2020;10(10):1870. doi: 10.3390/ani10101870 |
| 10. | Manspeaker M. Legal Investigations in Shelter Medicine. In: Byrd JH, Norris P, Bradley-Siemens N, eds. Veterinary Forensic Medicine and Forensic Sciences. 1st ed. CRC Press; Boca Raton FL, 2020:413–434. |
| 11. | Norris P. Animal Neglect and Abuse. In: Byrd JH, Norris P, Bradley-Siemens N, eds. Veterinary Forensic Medicine and Forensic Sciences. 1st ed. Boca Raton, FL: CRC Press; 2020:307–328. |
| 12. | Parmalee K. Crime Scene Investigation. In: Rogers ER, Stern AW, eds. Veterinary Forensics. 1st ed. Boca Raton, FL: CRC Press; 2018:23–52. |
| 13. | Touroo R, Fitch A. Identification, Collection, and Preservation of Veterinary Forensic Evidence. Vet Pathol. 2016;53(5):880–887. doi: 10.1177/0300985816641175 |
| 14. | Bradley-Siemens N. General Principles of Veterinary Forensic Sciences and Medicine. In: Byrd JH, Norris P, Bradley-Siemens N, eds. Veterinary Forensic Medicine and Forensic Sciences. 1st ed. Boca Raton, FL: CRC Press; 2020:21–34. |
| 15. | Merck MD. Crime Scene Investigation. In: Merck MD, ed. Veterinary Forensics: Animal Cruelty Investigations. 2nd ed. Oxford: John Wiley & Sons, Inc.; 2013:17–29. |
| 16. | Touroo R, Baucom K, Kessler M, Smith-Blackmore M. Minimum Standards and Best Practices for the Clinical Veterinary Forensic Examination of the Suspected Abused Animal. Forensic Sci Int Reports. 2020;2(June):100150. doi: 10.1016/j.fsir.2020.100150 |
| 17. | Davis G, McDonough S. Writing the Necropsy Report. In: Brooks J, ed. Veterinary Forensic Pathology. Vol. 2. Springer; 2018:139–150, Cham, Switzerland. |
| 18. | Rogers E, Stern A. Expert Witness Testimony and Report Writing. In: Rogers ER, Stern AW, eds. Veterinary Forensics. 1st ed. Boca Raton, FL: CRC Press; 2018:389–404. |
| 19. | Frederickson R. Demystifying the Courtroom. Vet Pathol. 2016;53(5):888–893. doi: 10.1177/0300985816647439 |
| 20. | McEwen B, Stern A, Viner T, et al. Veterinary Forensic Postmortem Examination Standards. Gainsville, FL; 2020. Accessed Aug 25, 2022. https://www.ivfsa.org/wp-content/uploads/2020/12/IVFSA-Veterinary-Forensic-Postmortem-Exam-Standards_Approved-2020_with-authors.pdf. |
| 21. | Bradley N, Smith-Blackmore M, Cavender A, Hirshberg E, Norris P. Standards Document for the Forensic Live Animal Examination. 2020. Accessed Aug 25, 2022. https://www.ivfsa.org/wp-content/uploads/2021/05/IVFSA_Veterinary-Forensic-Live-Animal-Exam-Standards_Approved-2020_With-authors.pdf. |
| 22. | Reisman RW. Medical Evaluation of Abused Live Animals. In: Miller L, Zawistowski SL, eds. Shelter Medicine for Veterinarians and Staff. 2nd ed. Oxford: John Wiley & Sons, Inc.; 2013:383–406. http://www.animallaw.info. |
| 23. | Stern A, Sula M-J. The Forensic Necropsy. In: Rogers ER, Stern AW, eds. Veterinary Forensics. 1st ed. Boca Raton, FL: CRC Press; 2018:109–152. |
| 24. | Brooks J. The Forensic Necropsy. In: Byrd JH, Norris P, Bradley-Siemens N, eds. Veterinary Forensic Medicine and Forensic Sciences. 1st ed. Boca Raton, FL: CRC Press; 2020:179–198. |
| 25. | Brownlie HWB, Munro R. The Veterinary Forensic Necropsy: A Review of Procedures and Protocols. Vet Pathol. 2016;53(5):919–928. doi: 10.1177/0300985816655851 |
| 26. | Merck M, Miller D, Maiorka P. CSI Examination of the Animal. In: Melinda M, ed. Veterinary Forensics: Animal Cruelty Investigations. 2nd ed. Ames, IA: Wiley-Blackwell; 2013:37–68. |
| 27. | Clark A. Animal Genetic Evidence and DNA Analysis. In: Byrd JH, Norris P, Bradley-Siemens N, eds. Veterinary Forensic Medicine and Forensic Sciences. 1st ed. Boca Raton, FL: CRC Press; 2020:57–66. |
| 28. | Smith-Blackmore M, Bradley-Seimens N. Animal Sexual Abuse. In: Byrd JH, Norris P, Bradley-Siemens N, eds. Veterinary Forensic Medicine and Forensic Sciences. 1st ed. Boca Raton, FL: CRC Press; 2020:113–128. |
| 29. | Norris P. Crime Scene Investigation. In: Byrd JH, Norris P, Bradley-Siemens N, eds. Veterinary Forensic Medicine and Forensic Sciences. 1st ed. Boca Raton, FL: CRC Press; 2020:1–20. |
| 30. | Woolf J, Brinker J. Forensic Physical Examination of the Cat and Dog. In: Ernest Rogers AWS, ed. Veterinary Forensics: Investigation, Evidence Collection, and Expert Witness Testimony. 1st ed. Boca Raton, FL: CRC Press; 2018:109–151. |
| 31. | Webb K. DNA Evidence Collection and Analysis. In: Rogers ER, Stern AW, eds. Veterinary Forensics. 1st ed. Boca Raton, FL: CRC Press; 2018:295–312. |
| 32. | Stern A, Blackmore-Smith M. Animal Sexual Abuse. In: Rogers ER, Stern AW, eds. Veterinary Forensics. 1st ed. Boca Raton, FL: CRC Press; 2018:349–362. |
| 33. | Merck M. Crime Scene Investigation. In: Merck MD, ed. Veterinary Forensics: Animal Cruelty Investigations. 2nd ed. West Sussex: John Wiley & Sons, Inc.; 2012:17–36. |
| 34. | Merck M, Miller D, Reisman R. Neglect. In: Merck MD, ed. Veterinary Forensics: Animal Cruelty Investigations. 2nd ed. West Sussex: John Wiley & Sons, Inc.; 2012:207–232. |
9. Behavior and Mental Well-Being
9.1 General
To promote animal health and well-being, it is essential for shelters to address emotional needs as well as physical needs.1–4 Emotional and behavioral needs are determined by environment, species, genetics, personality, prior socialization, and life experiences. Emotional and behavioral health have impacts on physical health, and vice versa. Shelters must provide behavioral care that considers the needs of individual animals as well as conditions experienced by the entire population.1,5
All shelter personnel should receive training about common behavior concerns at a level of detail appropriate to their position and job tasks. All relevant personnel must be trained in animal body language, objectively describing behavior, and how to interpret and respond to animal body language and behavior.6 Animals experiencing fear, anxiety, stress, and frustration are more likely to exhibit dangerous behaviors. Interactions that minimize negative mental states in animals improve handler safety, animal safety, and animal welfare.7 When interactions are positive, animals are more likely to accept and respond positively to additional interactions over time.8 Training in animal behavior allows personnel to recognize concerns and work to improve animal welfare.
9.2 Stress and welfare
Admission to a shelter is stressful for the vast majority of dogs and cats.9,10,11 Separation from caregivers, decreased and unfamiliar social interactions, confinement, loud noises, other stressed animals, and unpredictability all result in impaired welfare.12 Lack of control over one’s environment and separation from people are among the most profound stressors for companion animals.13 Shelters must have comprehensive protocols in place for recognizing and mitigating stress and associated negative emotions including fear, anxiety, and frustration.
Because confinement has negative impacts on animal behavior, reducing the duration of time spent in cages or kennels is critical. Foster care is generally the preferred method of housing for dogs and cats because it allows for regular social interaction and for animals to choose where and how they spend their time.14 When animals require care in a shelter facility (e.g. safety, legal, medical or behavioral reasons, or to facilitate adoptions), extra attention to well-being is necessary.
Animals must be monitored daily in order to detect trends or changes in well-being and respond to their behavioral needs. Actions must be taken to respond promptly to behavioral needs that impact welfare. When welfare is impaired, a health and behavior assessment is necessary to determine the severity of impairment and implement a plan to improve welfare. Any animal experiencing mental suffering, distress, or behavioral deterioration must be urgently assessed and treated.
Alternative housing and placement options must be urgently pursued for distressed animals not responding to behavioral care. Options include foster care, office foster, group housing, a different housing location, return to owner, or transfer to another shelter.15,16 However, for animals profoundly stressed by interactions with people, better options include return-to-field or placement in an appropriate environment (e.g. barn or warehouse). Distressed animals not responding to behavioral care should be humanely euthanized when other options are not feasible or available. When an animal is suffering and treatment efforts have failed, it is not appropriate or humane to postpone euthanasia in the hope that they will improve or another option will materialize.
9.3 Intake
Collecting information before admission allows the shelter to offer services that prevent intake, such as outpatient behavioral care, other rehoming resources, spay-neuter, or return-to-field. If intake to the shelter is necessary, personnel must collect a thorough behavioral history at or near the time of intake, including the reasons the animal was brought to the shelter and previously observed behavior. It is essential that personnel request information for every animal coming to the shelter, regardless of source.
A complete behavioral history is gathered by following a consistent process that collects key pieces of information, and additional details based on responses provided. Training in communication techniques assists intake personnel in completing this task, including asking open-ended questions, using objective language, and active listening. Available information about aggressive behavior must be recorded and include an objective description of the animal’s actions and the circumstances. Information about positive behaviors and preferences is also important. Personnel must use the available history to tailor animal care, meet the needs of individuals, and protect the safety and welfare of people and animals.
Shelters must work to minimize stress at the point of initial contact and throughout an animal’s stay. Functional separation of waiting areas, managed through scheduling or the use of partitions, placing carriers on elevated surfaces, and covering carriers with towels or blankets can reduce stress for incoming animals. Assessment of an animal’s behavior must begin at the time of first contact or intake and continue throughout their stay. The assessment process includes reviewing the history, observing behavior while in the shelter’s care, recording observations in the animal’s record, and communicating this information as needed.
9.4 Environmental management
The key to ensuring the best possible experience for animals living in the shelter is by creating an environment that minimizes stimuli that induce fear, stress, and frustration.5,17,18 Shelters must have policies and protocols for managing the environment in a manner that supports animal mental health and well-being. Understanding how dog and cat senses and cognition contribute to perception of the environment is an important part of environmental management (see Appendix E). Shelter housing and areas frequented by animals can be set up so that unwanted behaviors (e.g. barking and lunging) are less likely to occur than desired behaviors.19–21
9.4.1 Housing
Shelter housing has a tremendous impact on animal health and welfare (see Facilities). Novel environments are especially stressful for shy, under-socialized, or geriatric cats and dogs.1,10,22–24 Many animals benefit from foster care placement or housing in separate, calm, quiet areas beginning at intake. Feral animals must not be housed in the shelter except for a brief period of time related to the delivery of veterinary care.
Prey species must be housed away from predatory species at all times. Prey species (e.g. cats, birds, guinea pigs, hamsters, gerbils, and rabbits) become fearful and stressed when housed in olfactory, auditory, or visual contact with predatory species (e.g. ferrets, cats, and dogs). Cats not only are predators but may also be prey for dogs. Cats should not be handled or housed within spatial, visual, or auditory range of dogs.
9.4.2 Daily routine
Animals should be provided with a consistent and structured environment that minimizes reassignment of enclosures, caregivers, and schedules. An unpredictable environment can result in chronic fear and anxiety.13,25 Unpredictability includes a lack of routine in daily care, frequent disruption of enclosure set-up, as well as irregular patterns or continuous light or darkness.26 When events perceived as stressful are predictable, animals may experience periods of calm and relaxation between them because they learn what to expect.3 Animals also learn to look forward to positive experiences in their daily routines such as feeding and enrichment.
9.5 Enrichment and socialization
Enrichment refers to the process of improving the care of confined animals by providing them with:
- social interaction
- physical and mental stimulation
- opportunities to perform species-typical behaviors
- choice and control over their environment
Successful enrichment programs promote emotional well-being and minimize undesirable behaviors. Enrichment must be given the same significance as other components of animal care, such as nutrition and medical care, and is never considered optional. This is true whether animals are in a shelter facility or in a foster home. Positive social interaction, mental stimulation, and physical activity that meets each animal’s needs must be provided daily, outside of the activities of feeding and cleaning.
9.5.1 Time out of enclosure
Daily time out of the primary enclosure is one of the most effective means of reducing stress and frustration in kenneled dogs.27–29 Dogs must be provided with daily opportunities for activity outside of their kennels, unless doing so creates an unmanageable risk to the health or safety of people or other animals.
Cats must be offered regular opportunities to express natural behaviors, including physical activity and exploration. This can include time outside of their primary enclosure to exercise and explore in a secure, enriched setting. However, removal to a new location may not always be preferred or necessary for cats living in spacious, enriched rooms (especially with indoor-outdoor access).
For both dogs and cats, physical and mental activities outside of their enclosures need to be tailored to meet individual animal needs.
9.5.2 Interactions with people and other animals
Shelters should provide all animals with opportunities to engage in healthy social contact with people and other animals of the same species.13,30 Social isolation has a profoundly negative impact, and enrichment that meets the social needs of the animals is of the utmost importance in the shelter environment. Social interactions with people and other animals need to be monitored and individually tailored. For example, poorly socialized animals may not benefit from contact with people (with the exception of young puppies and kittens) but may find comfort in social interactions with their own species. Other animals, whether feral or socialized, may not enjoy interacting with members of their own species.
Regular positive daily social interaction with people is essential for all socialized dogs and cats, beginning at the time of admission. Providing appropriate daily social contact improves behavior, reduces defensive aggression, and supports physical health, particularly for fearful animals.8,31–33 Social contact with humans is essential even for animals with an unknown history or with an infectious disease concern. Positive social interactions with people, including calm, quiet interactions (e.g. sitting with or reading to) or more energetic play-centered interactions (e.g. wand, fetch, and tug) can be provided without removing the animal from the enclosure, if confinement is necessary for medical or behavioral reasons (Appendix F). Animals benefit greatly from having the opportunity to play, and play behavior is a strong indicator of positive welfare.5,34,35
9.5.3 Playgroups
Well-managed playgroup programs provide opportunities for healthy social contact with dogs and people. Playgroups require a safe and well-maintained space and the participation of sufficient personnel trained in canine behavior and humane handling.36 Selection and grouping of dogs based on health and behavior is necessary for safe, positive experiences.
Shelters should optimize human and animal safety by limiting the number of dogs in playgroups based on competency of personnel, play yard size, individual dog behavior, and shelter resources.36 Careful and consistent monitoring during playgroups and the use of humane techniques ensures participating dogs benefit from and enjoy the experience. Forcing dogs to interact when they have shown significant or consistent signs of fear, anxiety, or aggression increases the likelihood of defensive aggression, worsening fear, and injuries to dogs or personnel.
9.5.4 Enrichment within enclosures
Providing animals with an enriched primary enclosure is a critical aspect of sheltering. All cats need the opportunity to rest comfortably, hide, perch, scratch, play, and exercise choice within their environment. All dogs need the opportunity to rest comfortably, retreat from view, chew, play, and exercise choice within their environment. Shelters meet these needs by providing all animals with suitable housing, comfortable bedding, and toys. Scratching posts, elevated perches, and hiding boxes are also important for cats, while items to chew are also important for dogs.37,38 Feeding enrichment and olfactory, visual, auditory, and tactile stimulation can all be used as forms of sensory enrichment. It is important to provide animals with a rotation of novel enrichment items and activities to maintain interest (Appendix G).
9.5.5 Socialization of puppies and kittens
For young puppies and kittens, proper socialization with people and other animals of the same species is essential for normal behavioral development. Without daily gentle handling and positive exposure to a variety of novel stimuli, animals may develop chronic fear and anxiety, display aggressive behavior, or be unable to adjust normally to their environment. A broad range of positive socialization experiences must be provided to puppies and kittens and is best accomplished in a foster or adoptive home.
While in the shelter’s care, young puppies and kittens should be housed with their littermates and their mother. This interaction is important for normal behavioral and emotional development, as well as the establishment of species-specific behaviors. Single, unrelated puppies or kittens can greatly benefit from being housed with one or more age-matched individuals once health status for each is determined. Separation of puppies and kittens into pairs or smaller groups may be necessary to allow monitoring, completion of care tasks, foster placement, or to address medical or behavioral concerns.
9.6 Behavior assessment
In the shelter setting, the process of collecting information about an individual animal’s behavior is commonly referred to as ‘behavior assessment’. The goals of this process are to learn and interpret as much as possible about an individual animal’s behavior and use that information to:
- better understand the animal’s needs in the shelter and new home
- address behavior and welfare concerns
- match the animal with the appropriate outcome.39
Historically, a variety of methods have been used by shelters to assess behavior and prevent rehoming animals, especially dogs, who pose a public safety risk. This has included conducting behavior evaluation tests (i.e. temperament tests) where behavior is observed and interpreted in a structured format using a formal series of sub-tests performed one after the next (e.g. SAFER, Assess-a-Pet, and Match-up II).
Over the past two decades, studies have shown that behavior evaluation tests fail to reliably predict future behavior, particularly aggression, in a new home.40–43 Performing one stressful subtest after the next can negatively impact test results and the animal’s emotional well-being.8 For example, It is unacceptable to expose cats to dogs in the shelter as a test to determine if the dog can safely live with cats because this poses a significant risk of emotional and physical harm to cats. Formal testing requires considerable time and resources and can increase individual and population length of stay (LOS). For these reasons, requiring all shelter animals to go through a formal behavior evaluation test is no longer recommended.
Current recommendations for behavior assessment are to combine objective information collected via behavioral history with objective behavior observations noted during a variety of interactions.1,44,45 An overall behavior assessment must collect and consider all the information about the animal, including history and behaviors observed during all shelter and foster interactions. These interactions, with an emphasis placed on those likely to occur in a home setting, include intake procedures, daily care, medical handling and treatment, enrichment, play, and training activities, as well as interactions with personnel, visitors, adopters, and animals of the same species.
Through the process of behavior assessment, shelter personnel must strive to learn as much as possible about each animal to aid in optimizing their care, pathway planning, outcome decisions, and adoption matching and counseling. Training in current animal behavior science is necessary for personnel assessing shelter animal behavior, to give them the skills needed to reliably observe, document, assess, and act on findings or concerns. Documenting relevant behavior observations daily can track positive and negative trends in behavior and welfare. Behavior that requires intervention or affects how an animal can be safely handled must be entered into the animal’s record and communicated with shelter personnel promptly.
Behavior is highly influenced by stress, fear, and other negative emotional states as well as by the animal’s environment, previous experiences, and relationships with individual people and animals. When animals are experiencing high levels of stress or fear when interacting with people or other animals, they must not be forced to interact. In all cases, interactions with animals must not intentionally or carelessly provoke negative emotional states or undesirable behavior.
9.7 Responding to behavior or welfare concerns
When behavior or welfare concerns are present, it is important for shelters to develop an individualized plan, provide behavioral support, and make timely outcome decisions.
Environment modification and management to reduce undesirable behavior, as well as training, behavior modification, and behavior medications, can improve welfare and aid outcome and placement decisions.21 When deciding how to provide behavior support in the shelter, the impact on the animal, other animals in the shelter, shelter personnel, and future adopters require consideration. Behavior care and outcome decisions must be based on current animal behavior science. Approaches that increase length of stay in the shelter may result in unintended emotional deterioration or the development of new behavior problems. When behavior cannot be humanely managed in the shelter environment, seeking foster care and making timely outcome decisions are essential components of providing behavioral care.
9.7.1 Animal training
Animal training must be based on Least Intrusive Minimally Aversive principles and the Humane Hierarchy of Behavior Change in accordance with current professional guidelines.46,47 Positive reinforcement training programs for dogs and cats improve health, welfare, and likelihood of adoption.48–52 Training methods that incorporate punishment can increase fear, anxiety, and aggression toward people.21,53,54 These methods compromise both safety and welfare.55,56 Except when safety is an imminent concern, personnel should not use anything other than mildly aversive training methods. Ideally, animal trainers and behavior consultants are certified or have graduated from a program that assesses knowledge and skills.57
9.7.2 Behavior modification
Behavior modification applies techniques which change an animal’s behavior and underlying emotions. Behavior modification protocols must incorporate scientific principles of animal behavior and learning, such as classical conditioning, operant conditioning, and systematic desensitization and counterconditioning.21 It is unacceptable to use physical force as punishment to modify animal behavior.
Before implementing behavior modification, shelters must ensure they have the necessary resources to support such plans. Behavior modification is labor-intensive, time consuming, and must be applied consistently over a period of time in order to be successful. Behavior modification in the shelter environment may have a limited effect due to the significant impact of stress on animal behavior and learning. Placement in foster or an adopter’s home may facilitate response to the behavior modification plan.
9.7.3 Behavior medication
Behavior medications must be strongly considered to address welfare concerns related to emotional health. These medications may address immediate welfare concerns associated with shelter intake or housing, or long-term problems that impair welfare (e.g. separation anxiety, fear of people, and chronic stress associated with shelter housing). Behavioral health concerns must be objectively assessed and diagnosed to ensure that medications are prescribed when indicated, with clear goals for treatment and outcome.
Treatment goals include improving welfare, reducing stress and anxiety, and facilitating response to the behavioral treatment plan.21 Behavior medications must only be administered under the advice of or in accordance with written protocols provided by a veterinarian, and all drugs must be dispensed in accordance with federal and state regulations.
There are many alternative or complementary products also used to support animal behavior. In general, studies have been inconclusive or suggest minimal efficacy in shelter environments. It is the veterinarian’s duty to evaluate and consider the level of evidence for their use, and to weigh potential benefits against the shelter resources required.
When behavior medication is prescribed, it must be part of a comprehensive plan to help address the animal’s condition. This individually tailored comprehensive plan might include:
- continuing assessment (e.g. physical exam, diagnostic tests, and additional behavior assessment)
- environmental management
- daily routine adjustments
- foster care
- enrichment (additions or modifications)
- training or behavior modification
- complementary products and therapies
- monitoring response to treatment (e.g. medication and behavior modification)
9.7.4 Animals with long-term stays
Keeping length of stay as short as possible for each individual animal is a critical factor in maintaining animal welfare in shelters (see Population Management). For all animals staying in the shelter more than a few days, appropriate levels of additional enrichment must be provided on a daily basis. Chronic stress from prolonged stays in the shelter (i.e. more than 2 weeks) can reduce an animal’s ability to cope, increase fear, anxiety, and frustration, and underlie related behaviors such as social withdrawal, repetitive behaviors, and aggression. These behaviors can negatively impact other animals and personnel, and jeopardize placement options.9,13,58–64
In addition to more time and enrichment activities outside of their enclosures, housing that provides animals with additional space, enrichment, and choice within their enclosure must be provided for animals remaining in the shelter long-term. When an outcome is not quickly available (e.g. animals seized as legal evidence), foster care is a better choice than confinement in the shelter.15,65,66
Reproductive stress from estrous cycling and sex drive can decrease appetite, increase urine spraying, marking, and fighting, and profoundly increase social and emotional stress.67 Therefore, animals who are housed long-term should be spayed and neutered.
Long-term confinement of any animal who cannot be provided with basic care without inducing stress or compromising safety is unacceptable. Basic care includes daily enrichment and exercise. Feral animals, as well as those with persistent fear or aggressive behavior toward people, cannot be safely handled on a routine basis without inducing significant distress. These animals are unable to express natural and rewarding behavior, engage in play, or form social bonds in the shelter. Euthanasia is the humane option when live outcome (e.g. return-to-field) is not possible in a timely manner.
9.8 Risk assessment of animals displaying aggressive behavior
Shelters must promptly respond to behavior that poses a significant safety risk. When a dog or cat’s behavior might result in harm to people, other animals, or themselves, assessing the magnitude and likelihood of that harm is crucial.68,69 Risk assessment protocols provide a structured format, using all historical and current information gathered during behavior assessment, to make an educated estimate of an individual animal’s risk to the community and to determine whether that risk can be appropriately managed (see Table 9.1). The result of risk assessment is a comprehensive plan for reducing risk, including environmental and behavior management (which is often life-long) or euthanasia.
Shelters must have protocols and criteria in place that attempt to identify and manage animals at high risk of causing harm to shelter personnel, the public, or other domesticated animals. Decisions about rehoming require careful consideration of public safety, potential risks, and whether mitigation of these risks is feasible. Euthanasia is the appropriate outcome for animals at high risk of causing serious harm to people.
It is important for shelters to recognize that robust management efforts will not be suitable or sufficient to prevent aggressive incidents in every case or scenario, even when implemented thoroughly and consistently. Monitoring post-placement outcomes can help improve risk assessment processes. Consultation with legal professionals may be helpful when creating risk assessment and placement protocols for animals with a history of aggressive behavior.
9.9 Rehoming considerations
An important aspect of risk mitigation and supporting the quality of life for animals and people is providing resources and guidance to those who foster or adopt a shelter animal.39 Adopters and foster caregivers must be counseled on providing safe, gradual, and controlled introductions of shelter animals to children and resident pets.70 This helps create successful transitions and relationships. Foster caregivers and prospective adopters should be allowed to adopt or foster without bringing their own animals to the shelter.71 Information and counseling on strategies for safe and low-stress introductions can be tailored to the individual shelter or resident animal’s behavior and history.
A record of the animal’s behavior should be provided in hardcopy or electronic form with the animal at the time of transfer, foster, or adoption. When behavior concerns have been noted, communication about humane and appropriate management and modification of concerning behaviors reduces the risk of placing animals into a home environment and reduces shelter returns. Collecting post-adoption data regarding the success of behavior interventions helps shelters make needed adjustments and improves consensus within communities.
References
| 1. | Griffin B. Wellness. In: Miller L, Janeczko S, Hurley KF, eds. Infectious Disease Management in Animal Shelters. 2nd ed. Hoboken, NJ: Wiley Blackwell; 2021:13–45. |
| 2. | Mellor DJ, Beausoleil NJ. Extending the “Five Domains” Model for Animal Welfare Assessment to Incorporate Positive Welfare States. Anim Welf. 2015;24(3):241–253. doi: 10.7120/09627286.24.3.241 |
| 3. | McMillan FD. Development of a Mental Wellness Program for Animals. J Am Vet Med Assoc. 2002;220(7):965–972. doi: 10.2460/javma.2002.220.965 |
| 4. | McMillan FD, Vanderstichel R, Stryhn H, Yu J, Serpell JA. Behavioural Characteristics of Dogs Removed from Hoarding Situations. Appl Anim Behav Sci. 2016;178:69–79. doi: 10.1016/j.applanim.2016.02.006 |
| 5. | Kiddie JL, Collins LM. Development and Validation of a Quality of Life Assessment Tool for Use in Kennelled Dogs (Canis Familiaris). Appl Anim Behav Sci. 2014;158:57–68. doi: 10.1016/j.applanim.2014.05.008 |
| 6. | Lilly ML, Watson B, Siracusa C. Behavior Education and Intervention Program at a Small Shelter I. Effect on Behavior Knowledge and Safety. J Appl Anim Welf Sci. 2021;00(00):1–13. doi: 10.1080/10888705.2021.2012681 |
| 7. | Riemer S, Heritier C, Windschnurer I, Pratsch L, Arhant C, Affenzeller N. A Review on Mitigating Fear and Aggression in Dogs and Cats in a Veterinary Setting. Animals. 2021;11(1):1–27. doi: 10.3390/ani11010158 |
| 8. | Willen RM, Schiml PA, Hennessy MB. Enrichment Centered on Human Interaction Moderates Fear-Induced Aggression and Increases Positive Expectancy in Fearful Shelter Dogs. Appl Anim Behav Sci. 2019;217(March):57–62. doi: 10.1016/j.applanim.2019.05.001 |
| 9. | Stephen JM, Ledger RA. An Audit of Behavioral Indicators of Poor Welfare in Kenneled Dogs in the United Kingdom. J Appl Anim Welf Sci. 2005;8(June):79–95. doi: 10.1207/s15327604jaws0802 |
| 10. | Hennessy MB. Using hypothalamic-pituitary-adrenal measures for assessing and reducing the stress of dogs in shelters: A review. Appl Anim Behav Sci. 2013;149(1):1–12. doi: 10.1016/j.applanim.2013.09.004 |
| 11. | Tanaka A, Wagner DC, Kass PH, Hurley KF. Associations among Weight Loss, Stress, and Upper Respiratory Tract Infection in Shelter Cats. J Am Vet Med Assoc. 2012;240(5):570–576. doi: 10.2460/javma.240.5.570 |
| 12. | Lamon TK, Slater MR, Moberly HK, Budke CM. Welfare and Quality of Life Assessments for Shelter Dogs: A Scoping Review. Appl Anim Behav Sci. 2021;244:105490. doi: 10.1016/j.applanim.2021.105490 |
| 13. | Hennessy MB, Willen RM, Schiml PA. Psychological Stress, Its Reduction, and Long-Term Consequences: What Studies with Laboratory Animals Might Teach Us about Life in the Dog Shelter. 2020;10:2061. doi: 10.3390/ani10112061 |
| 14. | Gunter LM, Feuerbacher EN, Gilchrist RJ, Wynne CDL. Evaluating the Effects of a Temporary Fostering Program on Shelter Dog Welfare. PeerJ. 2019;2019(3):1–19. doi: 10.7717/peerj.6620 |
| 15. | Patronek GJ, Crowe A. Factors Associated with High Live Release for Dogs at a Large, Open-Admission, Municipal Shelter. Animals. 2018;8(4):1–15. doi: 10.3390/ani8040045 |
| 16. | Hoffman CL, Ladha C, Wilcox S. An Actigraphy-Based Comparison of Shelter Dog and Owned Dog Activity Patterns. J Vet Behav. 2019;34:30–36. doi: 10.1016/j.jveb.2019.08.001 |
| 17. | Ellis SLH, Rodan I, Carney HC, et al. AAFP and ISFM Feline Environmental Needs Guidelines. J Feline Med Surg. 2013;15(3):219–230. doi: 10.1177/1098612X13477537 |
| 18. | Yin S. Low Stress Handling, Restraint and Behavior Modification of Dogs and Cats. Davis, CA: Cattledog Publishing; 2009. |
| 19. | Bergman L, Gaskins L. Addressing Any Behavior Problem. Clin Brief. 2013;2:3. |
| 20. | Beugnet F, Bourdeau P, Chalvet-Monfray K, et al. Parasites of Domestic Owned Cats in Europe: Co-Infestations and Risk Factors. Parasites Vectors 2014;7(1):291. doi: 10.1186/1756-3305-7-291 |
| 21. | Overall KL. Feline behavior. In: Overall KL, ed. Manual of Clinical Behavioral Medicine for Dogs and Cats. 1st ed. St. Louis, MO: Elsevier; 2013. |
| 22. | Dybdall K, Strasser R, Katz T. Behavioral differences Between Owner Surrender and Stray Domestic Cats after Entering an Animal Shelter. Appl Anim Behav Sci. 2007;104(1–2):85–94. doi: 10.1016/j.applanim.2006.05.002 |
| 23. | Hiby EF, Rooney NJ, Bradshaw JWS. Behavioural and Physiological Responses of Dogs Entering Re-Homing Kennels. Physiol Behav. 2006;89(3):385–391. doi: 10.1016/j.physbeh.2006.07.012 |
| 24. | Slater M, Garrison L, Miller K, Weiss E, Drain N, Makolinski K. Physical and Behavioral Measures that Predict Cats’ Socialization in an Animal Shelter Environment During a Three Day Period. Animals. 2013;3(4):1215–1228. doi: 10.3390/ani3041215 |
| 25. | Carlstead K, Brown JJL, Strawn W. Behavioral and Physiological Correlates of Stress in Laboratory Cats. Appl Anim Behav Sci. 1993;38(2):143–158. doi: 10.1016/0168-1591(93)90062-T |
| 26. | Emmer K, Russart K, Walker W, Nelson R, DeVries AC. Effects of Light at Night on Laboratory Animals and Research Outcomes. Behav Neurosci. 2018;132(4):302–314. doi: 10.1037/bne0000252.Effects |
| 27. | Cafazzo S, Maragliano L, Bonanni R, et al. Behavioural and Physiological Indicators of Shelter Dogs’ Welfare: Reflections on the No-Kill Policy on Free-Ranging Dogs in Italy Revisited on the Basis of 15 Years of Implementation. Physiol Behav. 2014;133:223–229. doi: 10.1016/j.physbeh.2014.05.046 |
| 28. | Kiddie J, Collins L. Identifying Environmental and Management Factors that May Be Associated with the Quality of Life of Kennelled Dogs (Canis Familiaris). Appl Anim Behav Sci. 2015;167:43–55. doi: 10.1016/j.applanim.2015.03.007 |
| 29. | Protopopova A, Hauser H, Goldman KJ, Wynne CDLL. The Effects of Exercise and Calm Interactions on In-Kennel Behavior of Shelter Dogs. Behav Processes. 2018;146:54–60. doi: 10.1016/j.beproc.2017.11.013 |
| 30. | McMillan FD. The Psychobiology of Social Pain: Evidence for a Neurocognitive Overlap with Physical Pain and Welfare Implications for Social Animals with Special Attention to the Domestic Dog (Canis Familiaris). Physiol Behav. 2016;167:154–171. doi: 10.1016/j.physbeh.2016.09.013 |
| 31. | Gourkow N, Hamon SC, Phillips CJCC. Effect of Gentle Stroking and Vocalization on Behaviour, Mucosal Immunity and Upper Respiratory Disease in Anxious Shelter Cats. Prev Vet Med. 2014;117(1):266–275. doi: 10.1016/j.prevetmed.2014.06.005 |
| 32. | Gourkow N, Phillips CJC. Effect of Interactions with Humans on Behaviour, Mucosal Immunity and Upper Respiratory Disease of Shelter Cats Rated as Contented on Arrival. Prev Vet Med. 2015;121(3–4):288–296. doi: 10.1016/j.prevetmed.2015.07.013 |
| 33. | Gourkow N, Phillips CJC. Effect of Cognitive Enrichment on Behavior, Mucosal Immunity and Upper Respiratory Disease of Shelter Cats Rated as Frustrated on Arrival. Prev Vet Med. 2016;131:103–110. doi: 10.1016/j.prevetmed.2016.07.012 |
| 34. | Polgár Z, Blackwell EJ, Rooney NJ. Assessing the Welfare of Kennelled Dogs – A Review of Animal-Based Measures. Appl Anim Behav Sci. 2019;213:1–13. doi: 10.1016/j.applanim.2019.02.013 |
| 35. | Hunt RL, Whiteside H, Prankel S. Effects of Environmental Enrichment on Dog Behaviour: Pilot Study. Animals. 2022;12(2):1–8. doi: 10.3390/ani12020141 |
| 36. | Association of Shelter Veterinarians. Position Statement: Playgroups for Shelter Dogs. 2019. Accessed Dec 13, 2022. https://avsab.org/wp-content/uploads/2018/03/Punishment_Position_Statement-download_-_10-6-. |
| 37. | Ellis JJ, Stryhn H, Spears J, Cockram MS. Environmental Enrichment Choices of Shelter Cats. Behav Processes. 2017;141(April):291–296. doi: 10.1016/j.beproc.2017.03.023 |
| 38. | Van Der Leij WJR, Selman LDAM, Vernooij JCM, Vinke CM. The Effect of a Hiding Box on Stress Levels and Body Weight in Dutch Shelter Cats; A Randomized Controlled Trial. PLoS One. 2019;14(10):1–14. doi: 10.1371/journal.pone.0223492 |
| 39. | Reese LA. Make Me a Match: Prevalence and Outcomes Associated with Matching Programs in Dog Adoptions. J Appl Anim Welf Sci. 2021;24(1):16–28. doi: 10.1080/10888705.2020.1867985 |
| 40. | Patronek GJ, Bradley J. No Better than Flipping a Coin: Reconsidering Canine Behavior Evaluations in Animal Shelters. J Vet Behav Clin Appl Res. 2016;15:66–77. doi: 10.1016/j.jveb.2016.08.001 |
| 41. | Taylor KD, Mills DS. The effect of the kennel environment on canine welfare: a critical review of experimental studies. Anim Welf. 2007;16:435–447. |
| 42. | Mornement KM, Coleman GJ, Toukhsati S, Bennett PC. A Review of Behavioral Assessment Protocols Used by Australian Animal Shelters to Determine the Adoption Suitability of Dogs. J Appl Anim Welf Sci. 2010;13(4):314–329. doi: 10.1080/10888705.2010.483856 |
| 43. | Clay L, Paterson M, Bennett P, et al. In Defense of Canine Behavioral Assessments in Shelters: Outlining Their Positive Applications. J Vet Behav. 2020;38:74–81. doi: 10.1016/j.jveb.2020.03.005 |
| 44. | Ellis JJ. Feline Behavioral Assessment. In: Digangi BA, Cussen VA, Reid PJ, Collins KA, eds. Animal Behavior for Shelter Veterinarians and Staff. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2022:384–403. |
| 45. | Reid PJ. Assessing the Behavior of Shelter Dogs. In: Digangi BA, Cussen VA, Reid PJ, Collins KA, eds. Animal Behavior for Shelter Veterinarians and Staff. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2022:205–235. |
| 46. | International Association of Animal Behavior Consultants. IAABC Statement on LIMA. 2020. https://m.iaabc.org/about/lima/. |
| 47. | Blackwell EJ, Twells C, Seawright A, Casey RA. The Relationship between Training Methods and the Occurrence of Behavior Problems, as Reported by Owners, in a Population of Domestic Dogs. J Vet Behav Clin Appl Res. 2008;3(5):207–217. doi: 10.1016/j.jveb.2007.10.008 |
| 48. | Luescher AU, Tyson Medlock R. The Effects of Training and Environmental Alterations on Adoption Success of Shelter Dogs. Appl Anim Behav Sci. 2009;117(1–2):63–68. doi: 10.1016/j.applanim.2008.11.001 |
| 49. | Protopopova A, Wynne CDL. Adopter-Dog Interactions at the Shelter: Behavioral and Contextual Predictors of Adoption. Appl Anim Behav Sci. 2014;157:109–116. doi: 10.1016/j.applanim.2014.04.007 |
| 50. | Protopopova A, Mehrkam LR, Boggess MM, Wynne CDL. In-Kennel Behavior Predicts Length of Stay in Shelter Dogs. PLoS One. 2014;9(12):1–21. doi: 10.1371/journal.pone.0114319 |
| 51. | Gourkow N. Factors Affecting the Welfare and Adoption Rate of Cats in an Animal Shelter. Master’s Thesis, University of Calgary, 2001. |
| 52. | Grant RA, Warrior JR. Clicker Training Increases Exploratory Behaviour and Time Spent at the Front of the Enclosure in Shelter Cats; Implications for Welfare and Adoption Rates. Appl Anim Behav Sci. 2019;211(November 2018):77–83. doi: 10.1016/j.applanim.2018.12.002 |
| 53. | Deldalle S, Gaunet F. Effects of 2 Training Methods on Stress-Related Behaviors of the Dog (Canis Familiaris) and On the Dog-Owner Relationship. J Vet Behav Clin Appl Res. 2014;9(2):58–65. doi: 10.1016/j.jveb.2013.11.004 |
| 54. | Hiby EF, Rooney NJ, Bradshaw JWS. Dog Training Methods: Their Use, Effectiveness and Interaction with Behaviour and Welfare. Anim Welf. 2004;13(1):63–69. |
| 55. | Rooney NJ, Cowan S. Training Methods and Owner-Dog Interactions: Links with Dog Behaviour and Learning Ability. Appl Anim Behav Sci. 2011;132(3–4):169–177. doi: 10.1016/j.applanim.2011.03.007 |
| 56. | Arhant C, Bubna-Littitz H, Bartels A, Futschik A, Troxler J. Behaviour of Smaller and Larger Dogs: Effects of Training Methods, Inconsistency of Owner Behaviour and Level of Engagement in Activities with the Dog. Appl Anim Behav Sci. 2010;123(3–4):131–142. doi: 10.1016/j.applanim.2010.01.003 |
| 57. | International Association of Animal Behavior Consultants. Position Statement on Regulation in Animal Training and Behavior. Accessed Dec 13, 2022. https://m.iaabc.org/about/position-statements/regulation/. |
| 58. | Beerda B, Schilder MBH, Van Hooff JANARAM, De Vries HW, Mol JA. Chronic Stress in Dogs Subjected to Social and Spatial Restriction. I. Behavioral Responses. Physiol Behav. 1999;66(2):233–242. doi: 10.1016/S0031-9384(98)00289-3 |
| 59. | Wemelsfelder F. Animal Boredom: Understanding the Tedium of Confined Lives. In: McMillan FD, ed. Mental Health and Well-Being in Animals. Ames, IA: Blackwell Publishing Inc.; 2005:79–91. |
| 60. | Dalla Villa P, Barnard S, Di Fede E, et al. Behavioural and Physiological Responses of Shelter Dogs to Long-Term Confinement. Vet Ital. 2013;49(2):231–241. doi: 10.12834/VetIt.2013.492.231.241 |
| 61. | Denham H, Bradshaw J, Rooney NJ. Repetitive Behaviour in Kennelled Domestic Dog: Stereotypical or Not? Physiol Behav. 2014;128:288–294. doi: 10.1016/j.physbeh.2014.01.007 |
| 62. | Barnard S, Pedernera C, Candelora L, et al. Development of a New Welfare Assessment Protocol for Practical Application in Long-Term Dog Shelters. Vet Rec. 2016;178(1):18. doi: 10.1136/vr.103336 |
| 63. | Protopopova A. Effects of Sheltering on Physiology, Immune Function, Behavior, and the Welfare of Dogs. Physiol Behav. 2016;159:95–103. doi: 10.1016/j.physbeh.2016.03.020 |
| 64. | Raudies C, Waiblinger S, Arhant C. Characteristics and Welfare of Long-Term Shelter Dogs. Animals. 2021;11(1):1–21. doi: 10.3390/ani11010194 |
| 65. | Fehringer A, Dreschel NAA. Stress in Shelter Dogs and the Use of Foster Care to Improve Animal Welfare. J Vet Behav. 2014;9(6):e11. doi: 10.1016/j.jveb.2014.09.038 |
| 66. | Kerr CA, Rand J, Morton JM, Paterson M. Changes Associated with Improved Outcomes for Cats Entering RSPCA Queensland Shelters from 2011 to 2016. Animals. 2018;8(6):95. doi: 10.3390/ani8060095 |
| 67. | Griffin B, Hume K. Recognition and Management of Stress in Housed Cats. In: August J, ed. Consultations in Feline Internal Medicine. 5th ed. Philadelphia, PA: Elsevier Saunders; 2006:717–734. |
| 68. | van der Borg JAM, Beerda B, Ooms M, de Souza AS, van Hagen M, Kemp B. Evaluation of Behaviour Testing for Human Directed Aggression in Dogs. Appl Anim Behav Sci. 2010;128(1–4):78–90. doi: 10.1016/J.APPLANIM.2010.09.016 |
| 69. | Hunthausen WL. Assessing the Risk of Injury of Aggressive Dogs (Proceedings). DVM 360; 2009. Accessed Dec 13, 2022. https://www.dvm360.com/view/assessing-risk-injury-aggressive-dogs-proceedings-0. |
| 70. | Rayment DJ, De Groef B, Peters RA, Marston LC. Applied Personality Assessment in Domestic Dogs: Limitations and Caveats. Appl Anim Behav Sci. 2015;163:1–18. doi: 10.1016/j.applanim.2014.11.020 |
| 71. | Weiss E, Gramann S, Dolan ED, Scotto JE, Slater MR. Do Policy Based Adoptions Increase the Care a Pet Receives? An Exploration of a Shift to Conversation Based Adoptions at One Shelter. Open J Anim Sci. 2014;04(05):313–322. doi: 10.4236/ojas.2014.45040 |
10. Euthanasia
10.1 General
Maintaining positive welfare for animals in shelter care includes ensuring a humane death when euthanasia is appropriate. All animals and people must be treated with respect during the euthanasia process. Respect includes compassionate handling of the animal and its remains, consideration for the well-being of personnel involved, and compassionate interactions with those requesting euthanasia services. These recommendations apply whether euthanasia is performed in the shelter, the field, or a home setting.
The euthanasia process must be as free from pain, fear, anxiety, and distress as possible. Ensuring a humane death requires proper technique and expertise. To ensure euthanasia practices are suitable for each organization and the animals they serve, a veterinarian with appropriate training and expertise for the species involved should be consulted when establishing euthanasia protocols. Agents and methods deemed unacceptable in the AVMA Guidelines for the Euthanasia of Animals are unacceptable to use in shelters.1
Euthanasia decisions are based on the shelter’s ability to support the welfare of the individual animal in the context of the population, available resources, and the community. Rarely, there may be severe circumstances in which euthanasia of an entire population (i.e. depopulation) may be considered, such as in the event of a disease outbreak, disaster, or other population level crisis (see Medical Health). Depopulation must only be used as a last resort when all other methods to address the situation have been exhausted.2
10.2 Euthanasia process
Euthanasia protocols must be created and followed to support consistent euthanasia practices. Protocols include euthanasia drugs, delivery methods, handling plans, and environmental conditions. Protocols should have options to accommodate individual animal’s behavioral and physical needs and ensure human safety. Prompt intervention must occur if complications are noted during the euthanasia process. Complications could include delayed onset of sedation or death, excessive excitement, seizures, or vomiting. Adjustments to the euthanasia protocol may be needed if complications occur frequently.
It is unacceptable to euthanize an animal without confirming that the animal is the individual the shelter intends to euthanize. Using multiple methods to confirm an animal’s identity prior to euthanasia is important regardless of intake type. Shelter records, enclosure labels, collars, tags, physical descriptions, and people familiar with the animal may be consulted to ensure identification is correct. For stray animals, a final check of local missing animal listings should be performed to confirm that there are no matches before performing euthanasia.
Immediately prior to euthanasia, animals must be scanned for a microchip, either to confirm known microchip identity or in case previous scanning was incomplete. Multiple scans of the entire body using proper technique and a universal scanner maximize the chance of identifying a microchip.3 If a microchip is identified, ownership status requires follow-up before proceeding.
It is unacceptable to euthanize an animal without verifying legal eligibility. Legal eligibility includes verification that the organization owns or has legal responsibility for the animal (e.g. the animal is not on a court ordered or mandated stray hold), or the organization has consent from the animal’s owner, or the animal has a documented need for immediate euthanasia to alleviate suffering.
Performing euthanasia in the presence of other unfamiliar animals is not recommended because it may be stressful for animals in close proximity. However, when euthanasia is necessary for a litter of very young kittens or puppies, keeping them together during the euthanasia process may reduce the stress of separation. When the mother will also be euthanized, it is preferable to euthanize her first.
After the euthanasia procedure, death must be verified by trained staff before disposing of the animal’s body. The use of multiple verification methods is recommended. Lack of consciousness can be verified by the lack of blink reflex when the eye is touched, or the lack of response to a deep toe pinch. When breathing has stopped, cardiac standstill can be confirmed by the lack of movement of a needle inserted in the heart, or the lack of heartbeat using a stethoscope. Proper verification of death always includes confirmation of cardiac standstill or rigor mortis.1
10.2.1 Euthanasia methods
Euthanasia methods must be reliable, irreversible, compatible with the species, age, health and behavior of the animal, and ensure a smooth loss of consciousness followed by death. The use of pre-euthanasia sedation is generally recommended because it improves the experience for animals and personnel. Pre-euthanasia drugs must be administered when their use is necessary for a smooth euthanasia process. Their use is particularly important for animals who are in pain or are showing signs of fear, anxiety, or distress.
Each animal’s weight (actual or assessed) must be used to calculate adequate drug doses. The drugs and dosage used vary by drug availability4 and the chosen route of injection, whether intravenous (IV), intraperitoneal (IP), or intra-organ (including intrarenal or intracardiac). Each route of administration has benefits and drawbacks depending on the individual animal and circumstance. For example, IP injection is often the most humane strategy for very young or debilitated animals, while IV injection is preferred for pregnant animals. Unless an animal has been verified as unconscious, intra-organ injections are unacceptable.
While necessary in rare occasions in the field, gunshot is unacceptable as a routine method for euthanasia of dogs, cats, or other small companion animals.1 Inhalation of carbon monoxide is an unacceptable method of euthanasia for companion animals in shelters.5
10.3 Environment and equipment
A separate room should be designated for euthanasia in a quiet area away from the main pattern of foot traffic. The room used for euthanasia should be well lit and large enough to accommodate the necessary people and equipment. Only people with defined roles in the euthanasia process should be in the room when the procedure is being performed. These roles include technicians or veterinarians performing the euthanasia procedure and handling assistants, owners, familiar personnel, or trainees.
The euthanasia environment must be set up to minimize discomfort and distress and accommodate the individual animal’s behavioral and physical needs. Incorporating soft bedding, calm music, and comforting experiences (e.g. talking to the animal, gentle petting, toys, and food) is often beneficial for socialized animals. Other animals, such as wildlife and feral cats, are better served by minimal interaction and opportunities to hide.
All equipment used during the euthanasia process must be easily accessible and in good working order to ensure a safe and humane euthanasia process. A new needle must be used to administer euthanasia drugs to each animal because previously used needles may be dull or burred and cause unnecessary pain. Appropriate personal protective equipment must be utilized during the euthanasia process to avoid injury to personnel or transmission of disease. Euthanasia equipment and surfaces should be cleaned after each use, and the entire euthanasia room should be sanitized regularly.
All drugs used during the euthanasia process must be stored, administered, and documented in accordance with federal and state regulations. This includes keeping a record log documenting each animal’s identification, the amount of euthanasia solution and pre-euthanasia drugs used, the amounts remaining in the vial, and the identity of the person performing the euthanasia.6
Storage and final disposal of animal remains must be in compliance with all applicable laws and regulations. Proper storage is important to prevent disease transmission and unpleasant odors, and because medications, including those associated with euthanasia, may create a risk to scavenging animals. It is unacceptable for shelters to euthanize an animal solely for research or educational purposes. However, when shelter animals have already been euthanized for other reasons, and there is a clear benefit to other animals and society, their body may be used for science or teaching.7
10.4 Personnel considerations
Many states set training requirements and authorize who can perform euthanasia in shelters and under which circumstances. Veterinarians, veterinary technicians, animal control officers, and designated lay staff may be tasked with performing euthanasia in shelters.1 Personnel performing euthanasia must be appropriately trained and maintain all necessary certification as required by state or local regulations.
The safety and well-being of personnel must be incorporated into euthanasia protocols and policy. Because euthanasia is an important factor in the compassion fatigue, moral distress, and work-related strain reported by veterinarians and shelter staff,8,9 systems must be in place to prevent, recognize, and address fatigue and distress related to euthanasia in shelter personnel. This includes personnel involved in euthanasia decision-making, those performing the euthanasia procedure, and any who may be emotionally affected.8,10,11
Euthanasia decision-making must occur through a transparent process that lessens the decision-making burden on any one individual. Shelters can mitigate the stress of euthanasia on personnel by having clear and consistent decision-making protocols, sharing the decision-making burden, providing mentorship and training to those expected to perform euthanasia, rotating euthanasia performance duties, communicating transparently and sensitively about euthanasia, and holding debriefing sessions.12,13
References
| 1. | Leary S, Underwood W, Anthony R, et al. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. 2020th ed. Schaumburg, IL: American Veterinary Medical Association; 2020. |
| 2. | Association of Shelter Veterinarians. Position Statement: Depopulation. 2020. Accessed Dec 13, 2022. https://www.sheltervet.org/assets/docs/position-statements/DepopulationPS3.20.pdf. |
| 3. | Lord LK, Pennell ML, Ingwersen W, Fisher RA, Workman JD. In vitro sensitivity of commercial scanners to microchips of various frequencies. J Am Vet Med Assoc. 2008;233(11):1723–1728. doi: 10.2460/javma.233.11.1723 |
| 4. | Association of Shelter Veterinarians. Alternative euthanasia methods during pentobarbital sodium shortage. Accessed Dec 13, 2022. https://www.sheltervet.org/assets/PDFs/Euthanasiasolutionshortageinshelters_final.pdf. |
| 5. | Association of Shelter Veterinarians. Position statement: Euthanasia of shelter animals. 2020. Accessed Dec 13, 2022. https://www.sheltervet.org/assets/docs/position-statements/euthanasiaofshelteranimals.pdf. |
| 6. | U.S. Food & Drug Administration. Code of federal regulations title 21.9: Food and drugs. 2022. Accessed Dec 13, 2022. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=1304&showFR=1. |
| 7. | Association of Shelter Veterinarians. Position statement: Use of shelter animal cadavers for educational purposes. Accessed Dec 13, 2022. https://www.sheltervet.org/assets/docs/position-statements/CadaversPS2020.pdf. Published 2020. |
| 8. | Reeve CL, Rogelberg SG, Spitzmüller C, et al. The caring-killing paradox: Euthanasia-related strain among animal-shelter workers. J Appl Soc Psychol. 2005;35(1):119–143. doi: 10.1111/j.1559-1816.2005.tb02096.x |
| 9. | Tran L, Crane MF, Phillips JK. The distinct role of performing euthanasia on depression and suicide in veterinarians. J Occup Health Psychol. 2014;19(2):123–132. doi: 10.1037/a0035837 |
| 10. | Anderson KA, Brandt JC, Lord LK, Miles EA. Euthanasia in animal shelters: Management’s perspective on staff reactions and support programs euthanasia in animal shelters. Anthrozoos. 2015;26(4):569–578. doi: 10.2752/175303713X13795775536057 |
| 11. | Andrukonis A, Protopopova A. Occupational health of animal shelter employees by live release rate, shelter type, and euthanasia-related decision. Anthrozoos. 2020;33(1):119–131. doi: 10.1080/08927936.2020.1694316 |
| 12. | Jacobs J, Reese LA. Compassion fatigue among animal shelter volunteers: Examining personal and organizational risk factors. Anthrozoos. 2021;34(6):803–821. doi: 10.1080/08927936.2021.1926719 |
| 13. | Scotney RL, McLaughlin D, Keates HL. A systematic review of the effects of euthanasia and occupational stress in personnel working with animals in animal shelters, veterinary clinics, and biomedical research facilities. J Am Vet Med Assoc. 2015;247(10):1121–1130. doi: 10.2460/javma.247.10.1121 |
11. Animal Transport and Relocation Programs
11.1 General
Animal relocation programs involve the transfer and transport of animals from one sheltering organization (the source) to another (the destination). Transport can be local, regional, or international. The purpose is typically to move companion animals from communities with an excess pet population to communities with unmet adopter demand. Shelter animals are also relocated when they require services not available at the source shelter.
For many communities, relocation programs are a critical strategy to support live outcomes. However, relocation carries risks to health, behavior, and safety which can be particularly concerning for some animals.1–3 Intentionally designed relocation programs consider the risks and benefits for all affected animals and minimize negative impacts through careful selection and planning.
Decision-making in relocation programs must prioritize decreasing length of stay. Holding animals for relocation when live outcomes are available locally can lead shelters to operate beyond their capacity for care and compromise their relationship with their community4 (see Population Management). Likewise, when destination shelters accept more animals than they have the capacity to care for, the welfare of both relocated and destination animals may be compromised, and lengths of stay increased.
Shelters transport animals for a variety of reasons, such as local transfer, external medical services, enrichment activities, or relocation. It is unacceptable to transport animals when the transport itself is likely to be harmful to their immediate or long-term health or welfare. Careful management and planning are required to ensure that transporting an animal improves their welfare, and that priority is given to animal comfort and safety.
11.2 Responsibilities for relocation programs
All participants in the relocation process must follow federal regulations for animal transportation as well as local or state regulations for both source and destination locations. Departments of Agriculture and Departments of Health commonly have requirements for animals being imported into their jurisdiction. These often include health certificates (i.e. Certificates of Veterinary Inspection [CVI]) and certain vaccinations; there may also be restrictions for age and health conditions. For commercial air transport, organizations must consult with the airline for specific requirements.
Emergency plans must be made prior to transport. These plans include emergency contact information, safe locations to stop if necessary, protocols to address vehicle problems, and a plan for animal and human medical emergencies. Those transporting animals also need to have contact information for both the source and destination.
Clear direct communication is essential for successful relocation programs. Written agreements between all parties involved in the relocation program should be developed and reviewed regularly. Animal health and behavior must be accurately described and communicated between relocation partners. At minimum, such agreements address medical and behavioral selection criteria as well as transportation and destination requirements.5
A contact person must be identified at each transfer point, and a record of each animal’s travel from source to destination must be kept. Appropriate, accessible travel records allow tracing of an animal’s source and contacts along the route.
Public health and safety must be considered in the design of relocation programs and protocols. Zoonotic diseases with a regional distribution (e.g. plague, rabies, and Leptospirosis)6 and aggressive behaviors require special consideration (see Behavior, Public Health).
Organizations engaging in relocation should track standard metrics for transported animals. This includes animal demographics, behavioral and medical conditions, and outcomes.7 Unless there are extenuating circumstances, animals should not be returned to the source even in the event of unexpected medical or behavioral concerns. Transport is a significant stressor for the animal as well as a significant resource investment. If destination shelters regularly find that transported animals are not eligible for adoption, it is important for all parties to revisit selection criteria and program goals.
11.3 Responsibilities at the source
As with all shelters, all eligible animals within a source population must be vaccinated at or before intake8,9 (see Medical Health). It is insufficient to vaccinate only animals selected for relocation because it leaves the majority of animals unprotected. It is not recommended to hold animals back from transport just to allow response to vaccination or to receive a booster.10 To prevent the spread of internal and external parasites, treatment for fleas, ticks, and internal parasites is strongly recommended. Ideally, all dogs 6 months of age and older are tested for heartworm disease prior to relocation.11
The animal’s health and behavior records must be shared with the destination. When required, a valid health certificate (CVI) and proof of rabies vaccination must accompany each animal. Requirements may vary from state to state.
Animals must be examined by trained staff within 24 h prior to travel and deemed fit for transport. The purpose of the pre-transport examination is to look for evidence of infectious disease, and to evaluate the animal’s ability to tolerate the impact of the physical and emotional experiences encountered during travel (e.g. prolonged confinement, handling by multiple novel people, and direct exposure to other animals). A veterinarian must confirm that animals with medical concerns or recovering from surgery are fit for transport.
Animals being transported must be provided with visual identification. Collars or tags are routinely used, though in some cases, other techniques may be needed (e.g. marking the inner ear or painting a claw on a neonate). Ideally, animals are microchipped before transport, as this provides permanent identification. To aid in identification of individual animals, each primary transport enclosure must be marked with each animal’s unique identifier.
A copy of the manifest for each transport, identifying each animal on board, must be maintained in an accessible location separate from the vehicle itself, in case an accident leads to loss or destruction of the manifest accompanying the animals. For example, a cloud-based digital manifest can be made available to source, transporter, and destination in real time.
11.4 Responsibilities during transport
11.4.1 Primary enclosure and occupancy
For the safety and comfort of the animals, primary transport enclosures must be large enough for animals to stand and sit erect, turn around normally while standing, and lie in a natural position without lying on another animal. Unfamiliar animals must not be transported together in the same primary enclosure. Ideally, animals are introduced and acclimated to the transport carrier prior to transport in order to reduce associated stress.
The primary enclosure must not have sharp edges, and the flooring must prevent injury, discomfort, and leakage of fluids into other enclosures.12 To improve comfort and hygiene, absorbent bedding must be provided during transport unless it poses a risk to an individual animal’s health.
In a transport vehicle, kennels must be positioned in a manner that ensures adequate airflow and temperature regulation within each primary enclosure. Airflow is facilitated by choosing enclosures with vent openings on at least three sides, and maintaining at least 1 inch (2 cm) of unobstructed space between vent openings and adjacent structures. When primary enclosures are permanently fixed to the vehicle so that only a single door provides ventilation, the door needs to face an unobstructed aisle.12
Primary enclosures must be loaded in a manner that minimizes animal stress or discomfort while allowing direct visual observation. Primary enclosures must be secured to prevent movement within the vehicle, and doors secured to prevent accidental opening. In an emergency, operators must be able to swiftly remove animals.
11.4.2 Special cases
Cats
During transport, cats should be provided with a hiding space or visual barrier that allows ventilation and monitoring. For example, the kennel door can be partially covered with a towel, or a small hiding box can be provided within the primary enclosure. Stress can be further reduced if cats are acclimated to their carrier prior to transport and provided familiar objects with their own scent.13,14 Ideally, all cats are provided with access to a litter box during long-distance transport.
Cats and dogs are ideally transported in separate vehicles. If cats are transported in a vehicle with dogs, they must be housed in a physically separate space with special consideration given to visual and noise barriers.
Vulnerable populations
Puppies and kittens, geriatric animals, or animals with chronic medical or behavioral conditions require special care during transport. This care includes avoiding temperature extremes, more frequent feedings, and enhanced protection from infectious disease exposure during the transport process. Pediatric and brachycephalic animals are more susceptible to temperature extremes and may require different environmental parameters or alternative modes of transport.15,16 Kittens or puppies less than 8 weeks old should be transported with their mother when possible and should be transported in a single enclosure large enough for her to lie down with legs extended for comfort and to facilitate nursing. Importing animals under 8 weeks old may be prohibited in some states.
Sedation and behavior medication
Behavior medications should be considered when an animal is likely to have emotional welfare concerns during transport (see Behavior). Assessment of transport suitability is especially important for these animals. Clear communication between partners is essential when behavior medications are used. Safe and humane relocation programs do not use sedatives or behavior medications to compensate for poor transportation practices.
It is unacceptable for a relocation program to transport animals that are sedated or anesthetized to the point that they are unable to swallow, walk, or thermoregulate. Animals in this condition are at risk of choking, pneumonia, hypothermia, and cardiac and respiratory arrest without continuous monitoring by trained medical personnel.
11.4.3 Vehicles
Federal and local statutes for animal transport vehicles and their operation may not be sufficient to ensure humane care or the safety of animals and operators. Department of Transportation (DOT) regulations promote the safety of drivers and those around them and should be followed even when transporters are not licensed or subject to them. Vehicle operators must be licensed and trained in use of the specific vehicle they will be operating. Additional training in accident prevention and techniques to minimize animal discomfort during vehicle operation are recommended. For example, avoiding excessive lateral movement and sudden acceleration or deceleration are important skills to minimize animal stress and injury.
To ensure safe and humane conditions, control over heating and cooling in the animal compartment is essential in any vehicle used to transport animals.12 Interior temperatures of vehicles in direct sunlight can rapidly exceed safe levels, even when comfortable outside. The temperature of the animal compartment in the vehicle must be monitored, and action taken if low or high temperatures occur. Alarms can facilitate monitoring when drivers and animals are in separate compartments; placing the thermometer at the level of the animals allows for more accurate monitoring.
For animal safety, ambient temperature must be maintained above 45°F (7.2°C) and below 85°F (29.5°C), and humidity maintained between 30 and 70%.12,17 To ensure comfortable conditions, ambient temperature should be maintained between 64°F (18°C) and 80°F (26.6°C).17,18 Operators must ensure that air in the animal compartment is fresh and free of vehicle exhaust fumes.12 To detect poor air quality, carbon monoxide detectors should be placed in the animal compartment.
11.4.4 Monitoring and care
Vehicle drivers or animal attendants must have sufficient training in animal health, welfare, and safety to recognize and respond to animal needs during transport. For transports longer than 4 hours, two drivers should be present to monitor and reload animals. Having a second driver for longer trips allows one driver to rest while the other drives, or to assist in the case of an emergency. At minimum, every 4 hours, the vehicle must be stopped, and a visual observation of each animal must be performed.12
If it becomes necessary to remove animals from their enclosures for any reason, safeguards are needed to ensure animal safety and to prevent escape. For example, operators may have a supply of leashes, vehicles may be fitted with a secondary barrier around the exterior door, or protocols may specify closing exterior vehicle doors before opening primary enclosures.
Caregivers are charged with meeting the nutritional needs of transported animals. For juvenile animals, a small meal should be given no more than 4 hours before departure, and small amounts of food should be provided every 4 hours throughout transport. For both adults and juveniles, water must be provided at least every 4 hours during observation stops. Food must be provided at least every 24 hours for adult animals.12
Although federal regulations do not address travel distance for companion animals, risks to animal health and welfare generally increase with duration of transport.2 During transport, driving time to an intermediate or final destination should not exceed 12 hours per day, and loading and unloading of animals should not exceed 1 hour each (see Figure 11.1).15,19 Confinement for these lengths of time can still present welfare concerns, so efforts to reduce the overall transport duration, including stopping only when necessary and coordinating stops to manage both human and animal needs, are strongly recommended.
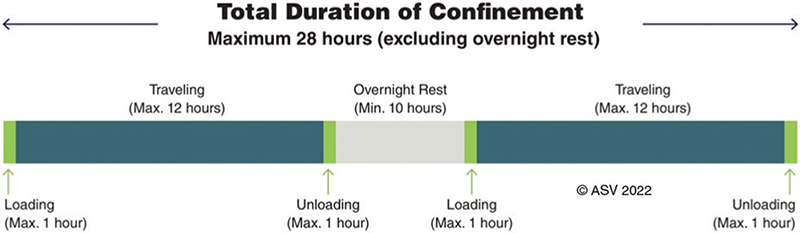
Figure 11.1. Maximum cumulative transport time to a final destination.
Transport that exceeds 12 hours of travel must be broken up with an overnight rest stop at an intermediary location. According to the DOT regulations for vehicle operators, overnight rest stops are at least 10 hours long. Total transport time from the source to a final destination should include no more than 28 hours confined to a transport vehicle, including loading and unloading time and excluding an overnight rest stop.12
Dogs must be walked or exercised on trips that require an overnight stay. Animals should never be left unattended in a transport vehicle unless sufficient monitoring capabilities are in place, and attendants are able to immediately respond to animal care needs. When feasible, an overnight facility that can accommodate the housing of cats and dogs off of the vehicle is preferred. Cats may benefit from remaining in their transport carriers, if large enough. Cats must have access to a litter box if being housed overnight. Overnight facilities can include foster homes, shelters, hotels, or transport hubs.
11.4.5 Aggregation
Safe and sustainable transport programs carefully manage different animal populations throughout the transport process. If transporting animals from different sources on separate vehicles is not possible, animals from each source are ideally housed in separate compartments. Whenever animals from different sources are held in the same vehicle or facility, protocols that minimize exposure and cross-contamination between populations must be in place.
11.5 Responsibilities at the destination
The destination shelter must have sufficient trained personnel ready to receive and evaluate animals upon arrival. Communication with transporters is important to ensure that the shelter has enough time to assemble their personnel. Each animal admitted through a relocation program must receive a brief health assessment at intake. This assessment identifies signs of infectious disease and problems that require emergency or follow-up medical care. Veterinary services must be accessible upon arrival. Access might include having a veterinarian on-site, on-call, or available at a local clinic.
The destination facility must have adequate housing prepared for the arriving animals without displacing the existing population. The need for isolation or quarantine of arriving animals is informed by regulatory requirements, animal health status, source organization practices, and infectious disease risk. Quarantines are only appropriate for high-risk animals with direct infectious disease exposure; unnecessary holds increase length of stay and are detrimental to animal health and organizational goals.
Destination shelters should maintain an active working knowledge of the source organization, which includes familiarity with the common diseases, preventive healthcare, and biosecurity practices at each source organization. Establishing procedures for continuing assessment, care, and communication after arrival promotes a healthy and successful partnership.
References
| 1. | Anderson MEC, Stull JW, Weese JS. Impact of dog transport on high-risk infectious diseases. Vet Clin North Am – Small Anim Pract. 2019;49(4):615–627. doi: 10.1016/j.cvsm.2019.02.004 |
| 2. | Aziz M, Janeczko S, Gupta M. Infectious disease prevalence and factors associated with upper respiratory infection in cats following relocation. Animals. 2018;8(6):1–11. doi: 10.3390/ani8060091 |
| 3. | Polak K. Dog transport and infectious disease risk: An international perspective. Vet Clin North Am – Small Anim Pract. 2019;49(4):599–613. doi: 10.1016/j.cvsm.2019.02.003 |
| 4. | DiGangi BA, Walsh KS. Behavioral care during transportation and relocation. In: DiGangi BA, Cussen V, Reid PJ, Collins K, eds. Animal Behavior for Shelter Veterinarians and Staff. 2nd ed. Hoboken, NJ: Wiley-Blackwell; 2022. |
| 5. | Doyle E. Medical aspects of companion animal transport programs. 2019. Accessed Dec 13, 2022. https://learning.theaawa.org/products/120419-medical-aspects-of-companion-animal-transport-programs. |
| 6. | White AM, Zambrana-Torrelio C, Allen T, et al. Hotspots of canine leptospirosis in the United States of America. Vet J. 2017;222:29–35. doi: 10.1016/j.tvjl.2017.02.009 |
| 7. | Shelter Animals Count. Basic data matrix. Accessed Oct 20, 2022. https://www.shelteranimalscount.org/wp-content/uploads/2022/02/BasicDataMatrix_SAC.pdf. |
| 8. | Stone A, Brummet GO, Carozza EM, et al. 2020 AAHA / AAFP feline vaccination guidelines. J Feline Med Surg. 2020;22:813–830. doi: 10.1177/1098612X20941784 |
| 9. | Ford RB, Larson LJ, Mcclure KD, et al. 2017 AAHA canine vaccination guidelines. 2017:26–35. Accessed Dec 13, 2022. https://www.aaha.org/public_documents/guidelines/vaccination_recommendation_for_general_practice_table.pdf. |
| 10. | Digangi BA, Craver C, Dolan ED. Incidence and predictors of canine parvovirus diagnoses in puppies relocated for adoption. Animals. 2021;11(4):1064. doi: 10.3390/ani11041064 |
| 11. | American Heartworm Society, Association of Shelter Veterinarians. Minimizing heartworm transmission in relocated dogs. 2017. Accessed Dec 13, 2022. https://www.sheltervet.org/assets/PDFs/Relocating%20HW%2BDogs.pdf |
| 12. | United States Department of Agriculture Animal and Plant Health Inspection Service. Code of federal regulations title 9.3.1: Specifications for the humane handling, care, treatment, and transportation of dogs and cats. 2021:47–128. Accessed Dec 13, 2022. https://www.ecfr.gov/current/title-9/chapter-I/subchapter-A/part-3. |
| 13. | Gruen MME, Thomson AE, Hamilton AK, et al. Conditioning laboratory cats to handling and transport. Lab Anim (NY). 2013;42(10):385–389. doi: 10.1038/laban.361 |
| 14. | Ellis SLH, Rodan I, Carney HC, et al. AAFP and ISFM feline environmental needs guidelines. J Feline Med Surg. 2013;15(3):219–230. doi: 10.1177/1098612X13477537 |
| 15. | American Veterinary Medical Association/Association of Shelter Veterinarians. Non-emergency relocation of dogs and cats for adoption within the United States: Best practices. 2020. Accessed Dec 13, 2022. www.avma.org › Reference › AVMA_BestPracticesAdoption_Brochure%0A. |
| 16. | Fitzgerald KT, Newquist KL. Husbandry of the neonate. In: Peterson ME, Kutzler MA, eds. Small Animal Pediatrics. St. Louis, MO: Elsevier Saunders; 2011:44–57. |
| 17. | National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals. In: Institute for Laboratory Animal Research, ed. ILAR’s Guide for the Care and Use of Laboratory Animals. 8th ed. National Academies Press; 2011. |
| 18. | American Veterinary Medical Association. AVMA policy: Companion animal care guidelines. Accessed Dec 13, 2022. https://www.avma.org/policies/companion-animal-care-guidelines. |
| 19. | National Federation of Humane Societies. Position statement: Best practices in animal transport protocols. Accessed Feb 4, 2020. http://www.humanefederation.org/TransferOverview.cfm. |
12. Disaster response
12.1 General
All shelters should be prepared to respond when directly affected by a disaster. Disasters include natural events such as hurricanes, tornadoes, floods, and fires, or human-made events such as large-scale cruelty cases, workplace violence, and toxic chemical spills. Advance planning is critical to safeguard animal welfare, and to protect human health and safety.1
Animal welfare needs described in this document are still present even when a shelter is experiencing a disaster. Deviations from these Guidelines as the result of a disaster should be as brief and as minimal as possible. Good planning helps ensure that these standards can be met under any circumstances. Additional published operational guidelines for animal evacuation & transport, animal decontamination, and emergency animal sheltering may be helpful in planning for and responding to disasters (Appendix H).
A disaster and its impacts may be localized to the shelter, the community it serves, or an entire region or country. Shelters outside of the impacted area may decide to offer aid to affected communities, including accepting and facilitating relocation of animals, sending personnel or resources, or providing advice and expertise. Whether impacted or offering aid, familiarity with disaster response principles is essential.
Disaster response is divided into four phases:
- Mitigation: on-going, preemptive activities that reduce the impacts of future disasters on animals, people, shelters, and communities
- Preparedness: creating plans to handle specific disasters, training and conducting exercises or drills, and acquiring the resources needed to respond
- Response: implementing the disaster plan and adapting as necessary during an event
- Recovery: returning to some degree of normalcy in the period following a disaster, this period can last from days to years
12.2 Mitigation
Shelters should take steps to anticipate, detect, and mitigate the impacts of disasters. In order for shelters to reduce the impact of a disaster, they must first identify the events most likely to affect them and their communities. Shelters must identify and plan for reasonably anticipated disasters, including those most likely to occur in their geographic area. Once disaster risks are identified, mitigation strategies can be developed and implemented to reduce the impact of a future disaster. Mitigation might include holding community pet identification and rabies vaccination clinics, reinforcing existing structures to better withstand common weather events, designing shelters according to building codes, and maintaining insurance and liability policies.
12.3 Preparedness
Every sheltering organization must have a written plan that outlines the actions the shelter will take in response to likely emergency scenarios. These actions may include services that the shelter does not typically provide, including admission of displaced animals, provision of resources, or relocation of animals to other facilities. The written disaster response plan should be accessible by all personnel, used to train staff during disaster drills, and regularly reviewed and updated.
Disaster response plans must detail how shelters will provide essential services to all animals currently in care, including those in foster homes. Essential services include sanitation, housing, food, and water as well as medical and behavioral care. Plans should detail how necessary supplies will be acquired, and include evacuation strategies in the event that supply chains or utilities (e.g. water, food, and heating or cooling) are disrupted.
Emergency plans should include a process for preemptively relocating the shelter’s population in advance of the event when appropriate. Evacuation ensures the safety of relocated animals and creates capacity to house and care for displaced community animals. Even if minimal animal intake from the community is expected, preemptive transport can reduce staffing challenges during a disaster and lessen the impact of facility damage on housed animals.
Since the risk of zoonotic disease spread may increase during disasters, plans must include steps to control transmission.2–4 These steps include providing wellness care, appropriate disease surveillance, and isolation and treatment of infected animals. Especially important during all disaster events is the consideration and control of rabies.3 Animal stress and anxiety leads to an increased likelihood of dog bites during disasters.2,4
Shelter disaster plans should indicate the personnel structure necessary to provide essential animal care services during a disaster. This structure identifies the critical personnel required and how the shelter plans to fill these roles. The staffing structure needs to be flexible, as animal care needs or personnel availability may be different than were anticipated. Critical personnel may be expected to perform new or additional roles or be recruited from outside organizations.
Training is an essential part of preparedness, as it is important for personnel to know what to do and when. Training specific to the roles personnel will fill during a disaster, including safety considerations, should be provided before starting the work. This training is best provided well in advance for personnel who are expected to respond to disasters but may be provided just prior to involvement. Even experienced personnel may need ‘just in time training’ in order to assume a new role.5 Exercises and drills are an excellent training tool and allow shelters to evaluate how well the current plan fits the organization’s needs.
Individuals participating in multi-agency disaster responses should complete National Response Framework (NRF) and National Incident Management System (NIMS) training, including Incident Command System (ICS) modules.5,6 These widely used systems provide a clear chain of command and communication structure, which can be scaled to meet the size and demands of any disaster.7 Partnerships are most successful when stakeholders are familiar with the shared vocabulary, operations, and processes guiding the response.8
Disasters are times of extreme stress for animals and people. Disaster planning should include provisions to address the physical and mental stress experienced by personnel, community members, and responders. Human safety must be the first priority of any disaster response plan.
Shelters can be key team members in coordinated community, state, or national disaster preparedness and response. If a shelter is part of an established disaster response team, a written plan should specify its particular role and the other organizations the shelter will be working with. Shelters responding to disasters as part of a coordinated response should draft memoranda of understanding (MOUs) with their governmental and nongovernmental response partners. MOUs enhance efficiency and secure resources by specifying which personnel, equipment, or facilities will be provided by each organization and clarifying roles and expectations.
12.4 Response
Response plans should be followed as soon as a disaster is anticipated or has occurred. Prompt response ensures critical shelter and community needs are addressed as quickly as possible. The most common challenge faced during a response is communication, both internally and externally.9,10 When indicated, an ICS should be initiated rapidly to designate and maintain a clear chain of command and communication infrastructure (see Appendix I).
Each animal admitted during a disaster must receive at least a cursory assessment at intake to check for signs of infectious disease, any conditions that require emergency medical care, and exposure to hazards. This allows staff to prioritize care where it is needed most and to separate animals to reduce the transmission of disease. Animals admitted during a disaster should be given core vaccines, including rabies and parasite control (see Medical Health).
Animals must be decontaminated when applicable (e.g. exposure to flood waters, fire retardants, or drug labs).11 Decontamination typically involves bathing and rinsing, with specific methods and products used depending on the potential contaminants.5,12–14 Because hazards on the animal may be a danger to animals and personnel, personal protective equipment (PPE) is recommended until decontamination is complete.
As soon as it is safe, shelters must make concerted efforts to reunify pets displaced by a disaster. Animal holding times (i.e. stray periods) and communication with owners may need to be broadened to reflect the challenges of the particular disaster. Using multiple methods to reach owners, including social media, flyers, electronic billboards, or neighborhood ambassadors, may be helpful in facilitating reunification. If an animal is transported out of the impacted area, clear communication between partner shelters regarding roles, processes, and timelines for reunification efforts is important.15
Shelters outside of the disaster area accepting impacted animals must be able to provide appropriate care and outcomes for their existing population before volunteering to accept displaced animals. Shelters are required to follow all relevant regulations and legal requirements related to animals even during disasters.
Shelters should have a system for managing physical and monetary donations during disaster response and recovery. Without a system, physical donations can become overwhelming and require valuable time and facilities to manage. Shelters should track resources used during disaster response and recovery. Detailed information, including staff time dedicated to response, may be requested for reimbursement grants from local, state, or federal agencies or private organizations.
Shelters must anticipate the arrival of self-deployed volunteers during a disaster and must address how these individuals will or will not be used.16 Volunteers may be unfamiliar with response plans and staffing structure, which can inadvertently place themselves and others at risk. However, preemptive planning for volunteer roles, training, and oversight can effectively mobilize this resource.
Responders may include volunteer veterinarians or veterinary technicians; veterinary professionals must only provide medical treatment or services when they hold a license to practice in that jurisdiction or are exempt from this requirement. Even during a disaster, oversight of use and storage of controlled substances must remain with the individual identified as the responsible party on the DEA license for that premise.
12.5 Recovery
The recovery period following a disaster lasts until the individual shelter and affected communities return to normal. Even if undamaged, shelters may be challenged by continuing impacts on their community or personnel. If damage to the shelter building, grounds, or local infrastructure is a concern, a full safety assessment must be made prior to resuming normal activities in that area or facility.
Shelters must tailor placement efforts when their community is impacted by a disaster. When local residents are struggling with rebuilding or finding shelter, fostering and adoption are unlikely to be a priority. Adoption events outside of the impacted community, increased shelter partner transfer, shelter-neuter-return, or other creative programs may help address longer lengths of stay.
Ongoing challenges during recovery may disproportionately impact some community members. Shelters should provide additional services that support keeping pets with their owners in the time frame immediately following the disaster. Sustained housing instability is a particular concern; shelters may be asked to assist an increased number of people facing eviction or displacement.17
Following a disaster, shelters should debrief and evaluate their planning, response, and recovery processes, so that adjustments to their plans can be made. The period of recovery from a disaster or major event is a natural time to broadly evaluate the effectiveness of programs, services, and procedures within the organization. Furthermore, shelters may decide to maintain changes implemented during the response that were valuable to the organization and community.
References
| 1. | Day AM. Companion animals and natural disasters: a systematic review of literature. Int J Disaster Risk Reduct. 2017;24:81–90. doi: 10.1016/j.ijdrr.2017.05.015 |
| 2. | CDC. Morbidity and mortality associated with hurricane loyd – North Carolina, September–October 1999. MMWR. 2000;49(17):369–372. Accessed Apr 1, 2022. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4917a3.htm. |
| 3. | CDC. Rabies in Manmade or Natural Disasters. 2011. Accessed Dec 13, 2022. https://www.cdc.gov/rabies/specific_groups/veterinarians/disasters.html |
| 4. | Mori J, Tsubokura M, Sugimoto A, et al. Increased incidence of dog-bite injuries after the Fukushima nuclear accident. Prev Med (Baltim). 2013;57(4):363–365. doi: 10.1016/j.ypmed.2013.06.013 |
| 5. | Center for Food Security & Public Health Iowa State University. Just-in-Time Training for Responders. Accessed Dec 13, 2022. http://www.cfsph.iastate.edu/Emergency-Response/just-in-time-training.php |
| 6. | Rogers C. The critical need for animal disaster response plans. J Bus Contin Emer Plan. 2015;9(3):262–271. |
| 7. | Green D. Chapter 2 - Incident Management. In: Animals in Disasters. First. St Louis, MO: Elsevier; 2019:9–20. doi: 10.1016/B978-0-12-813924-0.00002-5 |
| 8. | Wenzel JGW. Organizational aspects of disaster preparedness and response. J Am Vet Med Assoc. 2007;230(11):1634–1637. doi: 10.2460/javma.230.11.1634 |
| 9. | Green D. Chapter 1 - Introduction. In: Animals in Disasters. First. St Louis, MO: Elsevier; 2019:1–8. doi: 10.1016/B978-0-12-813924-0.00001-3 |
| 10. | A’Brunzo G, Bevan L, Garman EM, Lanham L, Schmitz J. Emergency Animal Sheltering Best Practices. 2009. |
| 11. | Gwaltney-Brant S. Managing animals seized from methamphetamine laboratory busts (Proceedings). DVM360 Magazine. Accessed Dec 13, 2022. https://www.dvm360.com/view/managing-animals-seized-methamphetamine-laboratory-busts-proceedings. |
| 12. | Centers for Disease Control and Prevention. Radiation Emergencies. Accessed Dec 13, 2022. https://www.cdc.gov/nceh/radiation/emergencies/. |
| 13. | Centers for Disease Control and Prevention. Radiation Safety: Removal of Radioactive Material (Decontamination). Accessed Dec 13, 2022. https://www.cdc.gov/nceh/radiation/decontamination.html. |
| 14. | Federal Emergency Management Administraton. Resource Typing Definition for Environmental Response/Health and Safety Emergency Response: Companion Animal Decontamination Team. 2018;(June):1–6. Accessed Dec 13, 2022. https://rtlt.preptoolkit.fema.gov/Public/Resource/ViewFile/1-508-1229?type=Pdf&q=animal. |
| 15. | Barron JF. Supporting Pet-to-Family Reunification in Disaster by Leveraging Human and Machine Computation. 2012. Accessed Dec 13, 2022. http://lse.summon.serialssolutions.com/link/0/eLvHCXMwY2BQSEm2NLM0SkxNszC1SDUzSDFMSTI3TTVPMgKtZDQ2Ae0bjgo3cfc2dQ4y9kEqzd1EGeTcXEOcPXRhpWJ8Sk5OvJGZJbBRDKynDQ3FGFiAneJUAJBOF9k |
| 16. | Irvine L. Ready or not: evacuating an animal shelter during a mock emergency. Anthrozoos. 2007;20(4):355–364. doi: 10.2752/089279307X245482 |
| 17. | Graham TM, Rock MJ. The spillover effect of a flood on pets and their people: implications for rental housing. J Appl Anim Welf Sci. 2019;22(3):229–239. doi: 10.1080/10888705.2018.1476863 |
13. Public Health
13.1 General
Public health promotes and protects people and the communities where they live, largely through One Health, which studies the connections among the well-being of animals, people, and the environment.1 The care that shelters provide to animals also impacts humans and the environment. Both within their facilities and in the larger community they serve, shelters must take precautions to protect the health and safety of animals, people, and the environment.
13.2 Personal protective measures
Shelter personnel encounter unavoidable risks to their health on a daily basis through normal work activities. Giving personnel the knowledge and equipment needed to mitigate risks is a critical component of workplace safety. Personal protective equipment (PPE) is worn to help prevent the spread of disease and to protect personnel from potentially harmful substances. In order to protect personnel from exposure to workplace hazards, shelters must provide PPE such as gloves, smocks, goggles, face masks, face shields, shoe covers, and ear plugs.2 PPE must be available in types and sizes to accommodate all personnel, including those with special concerns such as latex allergies.
13.2.1 Hand hygiene
Proper hand hygiene is essential to protecting human health in animal care environments. Personnel should wear gloves when handling animal waste or fluids and should wash hands frequently, especially after handling animals, and after removing PPE.3,4
Whether or not a person has had contact with animals, personnel should wash their hands before eating, smoking, or touching their face.5 As a precaution, personnel and visitors should be discouraged from eating, drinking, or bringing pacifiers, teething toys, or baby bottles into animal housing areas.3,6 To prevent the spread of zoonotic diseases, animals should not be present in areas designated for human food preparation or consumption.7
13.3 Workplace hazards
People working with and caring for animals are exposed to a diverse set of hazards. Shelters must comply with local, state, and federal health and safety regulations regarding chemical, biological, and physical hazards in the workplace.
13.3.1 Chemical hazards
Hazardous compounds, including disinfectants, medications, and pesticides, are routinely encountered in animal shelters.8 When working with hazardous chemicals, PPE such as eye protection or respirator face masks must be worn as indicated by the product label.9 A well-ventilated area or fume hood may also be required when working with certain products. Because mixing compounds such as bleach and ammonia can produce lethal toxic gas, Occupational Safety and Health Administration (OHSA) requires organizations to correctly label and store chemicals to prevent spills or accidental mixing.10–12
When allowed to accumulate or when improperly stored, animal urine and feces can become a significant source of toxic compounds such as ammonia and hydrogen sulfide.13–15 Shelters must promptly dispose of biological waste (animal waste, animal tissues, and carcasses) in a manner that follows state and local regulations.16,17
Shelters must follow regulatory guidelines for the disposal of unused medications.18,19 Controlled medications must be disposed of or wasted in a manner that follows regulations, prevents environmental contamination, and prevents human diversion.20 Guidance to reduce waste gas exposure associated with anesthesia may be found in the ASV’s Veterinary Medical Care Guidelines for Spay-Neuter Programs and from OSHA.21,22
Smoking must not be allowed in animal shelters. In addition to creating a risk of fire, second-hand smoke is harmful for pets and people.23–26
13.3.2 Physical hazards
Shelter personnel are also commonly exposed to physical hazards. These include slippery surfaces, loud noises such as barking or clanging metal, animal scratches and bites, job requirements to lift heavy objects and animals, and exposures to needles or other sharp objects.27 Shelters must follow industry guidelines for the proper disposal of sharps.28,29 Since the seriousness of physical injuries may initially be difficult to recognize, supervisors must advise persons injured at the shelter or by a shelter animal to seek medical care.
Noise exposure
Prolonged exposure to loud noise can damage the hearing of animals and people.30,31 Both environmental and behavioral noise abatement strategies should be used in animal housing and holding areas (see Facilities, Behavior). Hearing protection must be worn by employees working in environments where volume is at or above 100 dB cumulatively for 15 min. When volumes exceed 85 dB at any point in time, hearing protection should be worn.30,32 Several sound level meters are commercially available, including phone apps that measure decibel levels.33 Hearing conservation programs that include training and regular hearing testing may be required by OSHA depending on the average noise exposure.34 Hearing protection is recommended whenever personnel have to raise their voice in order to be heard three feet away.
13.3.3 Biological hazards
Animal bites
Animal bites are both a physical and biological hazard of significant concern in shelters. Training in animal body language, safe handling techniques, and the use of sedation can reduce but not eliminate the risk of bites (see Animal Handling). While many animal bites are minor, some are extremely serious with extensive tissue damage. All bites that break the skin carry a risk for infection, which can be reduced by immediately washing the wound.35 Deep penetrating punctures that close quickly, like those caused by cat bites, are at higher risk of developing a serious bacterial infection.36
The public must be prevented from having contact with animals who pose a high risk of biting by clearly marking and restricting access to areas where these animals are held. Shelters must consider public safety when making outcome decisions regarding animals who pose a risk of serious harm. If, after a careful, in-depth risk assessment, the shelter decides that an animal with a history of mild to moderate aggressive behavior is eligible for a live outcome (see Behavior), a record of all known bite incidents must be provided in hardcopy or electronic form to adopters, fosters, or transfer partners.
Human rabies exposure
Animal bites can transmit rabies virus. To allow for appropriate follow-up by public health authorities, shelters must follow regulations for reporting animal bites to humans.37 At intake, shelter personnel must ask owners or finders if the animal being admitted has bitten anyone within the past 10 days. Because aggression may be a symptom of rabies, animals who have bitten a human must be managed according to state and local regulations, including quarantine of the animal or euthanasia for rabies testing when required.38,39 Because animals who are symptomatic for rabies succumb to their illness within a week, the rabies quarantine period is typically 10 days.38,40 In some cases, euthanasia and testing may be preferred over quarantine, especially if the animal is suffering physically or emotionally, or presents a danger to others. If a dog, cat, or ferret dies for any reason within 10 days of a bite, testing for rabies is mandated. Local public health authorities can be contacted with questions about the management of other biting animals.
Because the consequences of rabies exposure are deadly, personnel who routinely work with animals should receive pre-exposure vaccinations against rabies in accordance with the current recommendations of the Advisory Committee on Immunization Practices.41
Animal rabies exposures
Shelters frequently admit animals with injuries or neurological symptoms of unknown cause. Though rare, these injuries or symptoms could be associated with rabies virus infection.42,43 At intake, shelter personnel must ask owners and finders of incoming animals about recent wildlife bites or exposures. During intake health assessments and physical examination, shelter personnel should look for and document evidence of wounds that could indicate a potential rabies exposure. Determining the appropriate quarantine period for an animal potentially exposed to rabies depends on species, previous rabies vaccination, and local regulations. Animals who have potentially been exposed to rabies must be managed with guidance from the NASPHV Rabies Compendium, and in accordance with state and local health regulations.38
Shelters should vaccinate all animals eligible for rabies vaccine prior to leaving the shelter44,45 (see Medical Health). Community cat vaccination is especially important because cats are the domestic animal most likely to acquire and transmit rabies in the United States and Canada.46–48
Other zoonotic diseases
Zoonotic diseases are transmitted from animals to people. Although all people are at risk of zoonotic disease, those with exposure to animals, and those with delayed or weakened immune responses due to young or old age, disease, pregnancy, or medical treatments have an increased risk.49,50 Not everyone is aware of their immune status or chooses to share this information. It is important that shelters implement policies that prevent, recognize, and manage zoonotic diseases.
Many common pathogens in the shelter can pass from animals to humans, including internal parasites (roundworms, hookworms, and toxoplasma), external parasites (mites), fungal diseases (ringworm), and bacterial diseases (Bordetella, Chlamydia, and Leptospira); viral diseases (rabies, influenza, and COVID-19) are less commonly transmitted to people. Even when the animal’s health is not significantly affected, timely treatment and management of animals with zoonotic pathogens help prevent spread to people and other animals.51
Training personnel to recognize zoonotic diseases is a key step in prevention.52 In addition to the general infectious disease control measures described in this document (see Medical Health), shelters should have a protocol for responding to zoonotic diseases, including communication regarding potential exposures. Reporting of some zoonotic diseases is mandated by local, state, and national regulations.
Access to animals with known zoonotic conditions should be limited to those necessary to provide appropriate care. Enclosures of animals with suspected zoonotic disease must be clearly marked to indicate the condition and necessary precautions, such as recommended PPE, handling, and sanitation practices. Shelters must disclose the risk of known zoonotic disease to personnel, transport partners, foster care providers, and adopters. Some states prohibit relocation of animals with zoonotic disease (see Animal Transport and Relocation Programs).
Antimicrobial resistance and emerging pathogens
Bacteria are continually evolving resistance to antibiotics. A key factor in slowing the development of resistance is to use antimicrobials only when truly needed.53 Routinely using antimicrobials to prevent infection in healthy animals is unacceptable.
Antimicrobial use must be tailored to appropriate clinical conditions, used judiciously, and evaluated for therapeutic effect.54–56 It is vital that antibiotics are only prescribed when they are effective against the pathogen of concern. To do this in a shelter, treatment protocols for common conditions need to be evidence-based and include specific criteria for diagnosis; which antibiotic, dosage, and duration to use; any follow-up considerations; and when to consult the veterinarian.57–60 Performing diagnostic testing is strongly recommended when animals do not respond to treatment or display unusual or severe signs of infection.61 When animals in shelters are managed in a manner that supports their physical and emotional health, the need for antimicrobial drugs is reduced.62,63
Some emerging diseases with the potential to infect people, such as influenza, were first identified in animal shelter populations.64,65 Because shelter populations can be sentinels for emerging diseases, animal shelters should monitor their populations for signs of unusual or severe disease. Poor sanitation practices, close housing of multiple species, housing diseased animals in the general population, and operating over capacity for care can facilitate the spread of disease.66 Animal population management should be used to reduce the risk of developing novel or emerging pathogens.
13.4 Human well-being
The well-being of shelter personnel is an important One Health concern. Both veterinarians and shelter employees have been shown to have high levels of compassion fatigue, secondary traumatic stress, moral injury, suicidal ideation, and burn-out as a result of their daily work.67–70 Shelters should strive to become workplaces that emphasize staff wellness through a positive organizational culture, fair pay, hours and expectations, provisions for self-care, and ready access to mental health support systems without repercussions. When mental health concerns are communicated or observed, personnel should be encouraged to seek professional help.71
Being able to provide appropriate care to shelter animals, and seeing their quality of life improve as a result of that care, can also reduce work-related stress for shelter personnel.72,73 In turn, personnel who are satisfied with their work are more likely to provide high-quality care for animals and stay in the workforce.73,74 Providing personnel with the skills, resources, and authority to excel at their jobs creates a beneficial cycle, improving human, animal, and population health.
References
| 1. | Centers for Disease Control and Prevention. One Health Basics. National Center for Emerging and Zoonotic Infectious Diseases. 2018. Accessed Dec 13, 2022. https://www.cdc.gov/onehealth/basics/index.html |
| 2. | Occupational Health and Safety Administration. Employers Must Provide and Pay for PPE. 2017;(April):1–2. Accessed Dec 13, 2022. https://www.osha.gov/sites/default/files/Handout_2_Employers_Must_Provide_and_Pay_for_PPE.pdf |
| 3. | Centers for Disease Control and Prevention. Proper Hygiene When Around Animals. Accessed Dec 13, 2022. https://www.cdc.gov/healthywater/hygiene/etiquette/around_animals.html |
| 4. | Centers for Disease Control and Prevention. When and How to Wash Your Hands. Accessed Dec 13, 2022. https://www.cdc.gov/handwashing/when-how-handwashing.html. |
| 5. | Centers for Disease Control and Prevention. Hand Hygiene at Work. Accessed Dec 13, 2022. https://www.cdc.gov/handwashing/handwashing-corporate.html |
| 6. | Smith K, Dunn J, Castrodale L, Wohrle R. Compendium of measures to prevent disease associated with animals in public settings, 2013. Javma. 2016;248(5):1997–2001. doi: 10.2460/javma.248.5.505 |
| 7. | Food and Drug Administration: Public Health Service. FDA Food Code. College Park MD; 2017. Accessed Dec 13, 2022. http://www.cgdev.org/sites/default/files/More-Health-for-the-Money.pdf%5Cnpapers3://publication/uuid/2A00668B-CF93-4560-B974-A6AC1DBED31B |
| 8. | Thomann WR. Chemical safety in animal care, use, and research. ILAR J. 2003;44(1):13–19. doi: 10.1093/ilar.44.1.13 |
| 9. | National Institute for Occupational Safety and Health. NIOSH Pocket Guide to Chemical Hazards. No. 2005-1. Cincinnati OH: NIOSH Publications; 2007. doi: 10.1109/icnn.1993.298588 |
| 10. | Occupational Safety and Health Administration. Chemical Hazards and Toxic Substances. Accessed Dec 13, 2022. https://www.osha.gov/chemical-hazards |
| 11. | Washington State Department of Health. Dangers of Mixing Bleach with Cleaners. Accessed Dec 13, 2022. https://doh.wa.gov/community-and-environment/contaminants/bleach-mixing-dangers |
| 12. | Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health. Protecting Workers Who Use Cleaning Chemicals. 2012:1–3. Accessed Dec 13, 2022. http://www.epa.gov/oppad001/ad_info.htm%0Ahttps://www.osha.gov/Publications/OSHA3512.pdf |
| 13. | Mielke SR. A Pilot Study of Potential Public Health Hazards in the Animal Hoarding Environment. 2015. Accessed Dec 13, 2022. http://rave.ohiolink.edu/etdc/view?acc_num=osu1429707141 |
| 14. | Neghab M, Mirzaei A, Shouroki FK, Jahangiri M, Zare M, Yousefinejad S. Ventilatory disorders associated with occupational inhalation exposure to nitrogen trihydride (Ammonia). Ind Health. 2018;56(5):427–435. doi: 10.2486/indhealth.2018-0014 |
| 15. | Kirkhorn SR, Garry VF. Agricultural lung diseases. Environ Health Perspect. 2000;108(suppl. 4):705–712. doi: 10.1289/ehp.00108s4705 |
| 16. | Center for Disease Control. Workbook for Designing, Implementing, and Evaluating a Sharps Injury Prevention Program. Vol VI.; 2008. |
| 17. | Environmental Protection Agency. Medical Waste. Accessed Dec 13, 2022. https://www.epa.gov/rcra/medical-waste |
| 18. | Food and Drug Administration. Disposal of Unused Medicines: What You Should Know. Accessed Dec 13, 2022. https://www.fda.gov/drugs/safe-disposal-medicines/disposal-unused-medicines-what-you-should-know |
| 19. | Environmental Protection Agency. How to Dispose of Medicines Properly. 2011;816-F-11-0:2. Accessed Dec 13, 2022. https://archive.epa.gov/region02/capp/web/pdf/ppcpflyer.pdf |
| 20. | Code of Federal Regulations. Code of Federal Regulations Title 21.2.1317: Dosposal of Controlled Substances by Registrants. 2021. Accessed Dec 13, 2022. https://www.ecfr.gov/current/title-21/chapter-II/part-1317 |
| 21. | Griffin B, Bushby PA, Mccobb E, et al. The Association of Shelter Veterinarians’ 2016 Veterinary Medical Care Guidelines for Spay-Neuter Programs. J Am Vet Med Assoc. 2016;249(2):165–188. |
| 22. | Occupational Safety and Health Administration. Anesthetic Gases: Guidelines for Workplace Exposures. 2020. Accessed Dec 13, 2022. https://www.osha.gov/waste-anesthetic-gases/workplace-exposures-guidelines |
| 23. | Centers for Disease Control and Prevention. Smoking & Tobacco Use: Fast Facts and Fact Sheets. Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion. |
| 24. | Seguel JM, Merrill R, Seguel D, Campagna AC. Indoor Air Quality. Am J Lifestyle Med. 2017;11(4):284–295. doi: 10.1177/1559827616653343 |
| 25. | Bertone ER, Snyder LA, Moore AS. Environmental tobacco smoke and risk of malignant lymphoma in pet cats. Am J Epidemiol. 2002;156(3):268–273. doi: 10.1093/aje/kwf044 |
| 26. | Roza MR, Viegas CAA. The dog as a passive smoker: Effects of exposure to environmental cigarette smoke on domestic dogs. Nicotine Tob Res. 2007;9(11):1171–1176. doi: 10.1080/14622200701648391 |
| 27. | Fowler H, Adams D, Bonauto D, Rabinowitz P. Work-related injuries to animal care workers, Washington 2007–2011. Am J Ind Med. 2016;59(3):236–244. doi: 10.1002/ajim.22547 |
| 28. | U.S. Food & Drug Administration. DOs and DON’Ts of Proper Sharps Disposal. 2011;4(1):1–2. Accessed Dec 13, 2022. https://www.fda.gov/medical-devices/safely-using-sharps-needles-and-syringes-home-work-and-travel/dos-and-donts-proper-sharps-disposal. |
| 29. | Center for Disease Control and Prevention. National Occupational Research Agenda. Stop Sticks Campaign. 2019. Accessed Dec 13, 2022. https://www.cdc.gov/nora/councils/hcsa/stopsticks/default.html. |
| 30. | Occupational Health and Safety Administration. Occupational Noise Exposure. Accessed Dec 13, 2022. https://www.osha.gov/noise |
| 31. | Scheifele P, Martin D, Clark JG, Kemper D, Wells J. Effect of kennel noise on hearing in dogs. Am J Vet Res. 2012;73(4):482–489. doi: 10.2460/ajvr.73.4.482 |
| 32. | National Institute for Occupational Safety and Health (NIOSH). Hearing Loss Prevention Program. 2018:1. Accessed Dec 13, 2022. http://www2.worksafebc.com/topics/hearinglossprevention/HearingLossPreventionProgram.asp |
| 33. | Center for Disease Control and Prevention, National Institute for Occupational Safety and Health. NIOSH Sound Level Meter App. 2022. Accessed Dec 13, 2022. https://www.cdc.gov/niosh/topics/noise/app.html |
| 34. | Occupational Safety and Health Administration. Hearing Conservation. 1st ed. Washington, DC: U.S. Department of Labor; 2002. |
| 35. | Elcock KL, Reid J, Moncayo-Nieto OL, Rust PA. Biting the hand that feeds you: management of human and animal bites. Injury. 2022;53(2):227–236. doi: 10.1016/j.injury.2021.11.045 |
| 36. | Ellis R, Ellis C. Dog and Cat Bites (corrected). Am Fam Physician. 2014. Accessed Dec 13, 2022. https://www.aafp.org/afp/2014/0815/p239.html. |
| 37. | Center for Disease Control and Prevention, National Occupational Research Agenda. What to Do with an Animal that has Bitten a Person. 2022. Accessed Dec 13, 2022. https://www.cdc.gov/rabies/specific_groups/veterinarians/person_bitten.html |
| 38. | Brown CM, Slavinski S, Ettestad P, Sidwa TJ, Sorhage FE. Compendium of animal rabies prevention and control. J Am Vet Med Assoc. 2016;248(5):505–517. |
| 39. | Centers for Disease Control and Prevention. When Should I Seek Medical Attention? 2022. Accessed Dec 13, 2022. https://www.cdc.gov/rabies/exposure/index.html |
| 40. | Lackay SN, Yi K, Zhen FF. Rabies in small animals. Vet Clin North Am Small Anim Pr. 2008;38(4):851-ix. |
| 41. | Rao AK, Briggs D, Moore SM, et al. Use of a modified preexposure prophylaxis vaccination schedule to prevent human rabies: Recommendations of the advisory committee on immunization Practicesp – United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(18):619–627. doi: 10.15585/mmwr.mm7118a2 |
| 42. | Fogelman V, Fischman H, Horman J, Grigor J. Epidemiologic and clinical characteristics of rabies in cats. J Am Vet Med Assoc. 1993;202(11):1829–1833. |
| 43. | Singh R, Singh KP, Cherian S, et al. Rabies – epidemiology, pathogenesis, public health concerns and advances in diagnosis and control: a comprehensive review. Vet Q. 2017;37(1):212–251. doi: 10.1080/01652176.2017.1343516 |
| 44. | Stone A, Brummet GO, Carozza EM, et al. 2020 AAHA/AAFP feline vaccination guidelines. J Feline Med Surg. 2020;22:813–830. doi: 10.1177/1098612X20941784 |
| 45. | Chomel BB, Sykes JE. Rabies. In: Sykes JE, ed. Greene’s Infectious Diseases of the Dog and Cat. 5th ed. St Louis, MO: Elsevier Health Sciences; 2022:260–270. |
| 46. | Ma X, Monroe B, Wallace RM, et al. Rabies surveillance in the United States during 2019. J Am Vet Med Assoc. 2021;258(11):1205–1220. |
| 47. | Frymus T, Addie D, Belak S, et al. Feline rabies: ABCD guidelines on prevention and management. J Feline Med Surger. 2009;11:585–593. |
| 48. | Levy JK, Wilford CL. Management of stray and feral community cats. In: Miller L, Zawistowski SL, eds. Shelter Medicine for Veterinarians and Staff. 2nd ed. Ames, IA; John Wiley & Sons. 2013:669–688. |
| 49. | Stull JW, Stevenson KB. Zoonotic disease risks for immunocompromised and other high-risk clients and staff: promoting safe pet ownership and contact. Vet Clin North Am – Small Anim Pract. 2015;45(2):377–392. doi: 10.1016/j.cvsm.2014.11.007 |
| 50. | The National Association of State Public Health Veterinrians Veterinary Infection Control Committee. Compendium of veterinary standard precautions for zoonotic disease prevention in veterinary personnel. J Am Vet Med Assoc. 2015;247(11):1254–1276. |
| 51. | Babbitt J. Operational Guide for Animal Care and Control Agencies: Companion Animal Zoonotic Diseases. 2010:1-47. |
| 52. | Steneroden KK, Hill AE, Salman MD. Zoonotic disease awareness in animal Shelter Workers and volunteers and the effect of training. Zoonoses Public Health. 2011;58(7):449–453. doi: 10.1111/j.1863-2378.2011.01389.x |
| 53. | Lloyd DH, Page SW. Antimicrobial stewardship in veterinary medicine. Microbiol Spectr. 2018;6(3). doi: 10.1128/microbiolspec.arba-0023-2017 |
| 54. | American Veterinary Medical Association. Policy: Antimicrobial Stewardship Definition and Core. Accessed Dec 13, 2022. https://www.avma.org/resources-tools/avma-policies/antimicrobial-stewardship-definition-and-core-principles |
| 55. | American Veterinary Medical Association. Policy: Antimicrobial Use Guidelines for Veterinary Practice. Accessed Dec 13, 2022. https://www.avma.org/resources-tools/avma-policies/antimicrobial-use-guidelines-veterinary-practice |
| 56. | American Association of Feline Practitioners, American Animal Hospital Association. Basic Guidelines of Judicious Therapeutic Use of Antimicrobials. 2006;(January):1–5. |
| 57. | Lappin MR, Blondeau J, Boothe D, et al. Antimicrobial use guidelines for treatment of respiratory tract disease in dogs and cats: antimicrobial guidelines working group of the International Society for Companion Animal Infectious Diseases. J Vet Intern Med. 2017;31(2):279–294. doi: 10.1111/jvim.14627 |
| 58. | Papich MG. Antibiotic treatment of resistant infections in small animals. Vet Clin North Am – Small Anim Pract. 2013;43(5):1091–1107. doi: 10.1016/j.cvsm.2013.04.006 |
| 59. | Nelson LL. Surgical site infections in small animal surgery. Vet Clin North Am – Small Anim Pract. 2011;41(5):1041–1056. doi: 10.1016/j.cvsm.2011.05.010 |
| 60. | Weese JS, Blondeau JM, Boothe D, et al. Antimicrobial use guidelines for treatment of urinary tract disease in dogs and cats: antimicrobial guidelines working group of the international society for companion animal infectious diseases. Vet Med Int. 2011;2011: 1–9. doi: 10.4061/2011/263768 |
| 61. | Allerton F, Nuttall T. Antimicrobial use: importance of bacterial culture and susceptibility testing. In Pract. 2021;43(9):500–510. doi: 10.1002/inpr.139 |
| 62. | Gourkow N, Hamon SC, Phillips CJCC. Effect of gentle stroking and vocalization on behaviour, mucosal immunity and upper respiratory disease in anxious shelter cats. Prev Vet Med. 2014;117(1):266–275. doi: 10.1016/j.prevetmed.2014.06.005 |
| 63. | Hennessy MB, Willen RM, Schiml PA. Psychological stress, its reduction, and long-term consequences: what studies with laboratory animals might teach us about life in the dog shelter. Animals (Basel)2020;10(11):2061. doi: 10.3390/ani10112061 |
| 64. | Lee CT, Slavinski S, Schiff C, et al. Outbreak of influenza A ( H7N2 ) among cats in an animal shelter with cat-to-human transmission – New York City, 2016. Clin Infect Dis Br Rep. 2017;24:1927–1929. doi: 10.1093/cid/cix668 |
| 65. | Anderson TC, Bromfield CR, Crawford PC, Dodds WJ, Gibbs EPJ, Hernandez JA. Serological evidence of H3N8 canine influenza-like virus circulation in USA dogs prior to 2004. Vet J. 2012;191(3):312–316. doi: 10.1016/j.tvjl.2011.11.010 |
| 66. | Pesavento PA, Murphy BG. Common and emerging infectious diseases in the animal shelter. Vet Pathol. 2014;51(2):478–491. doi: 10.1177/0300985813511129 |
| 67. | Jacobs J, Reese LA. Compassion fatigue among animal shelter volunteers: examining personal and organizational risk factors. Anthrozoos. 2021;34(6):803–821. doi: 10.1080/08927936.2021.1926719 |
| 68. | Scotney RL, McLaughlin D, Keates HL. A systematic review of the effects of euthanasia and occupational stress in personnel working with animals in animal shelters, veterinary clinics, and biomedical research facilities. J Am Vet Med Assoc. 2015;247(10):1121–1130. doi: 10.2460/javma.247.10.1121 |
| 69. | Andrukonis A, Protopopova A. Occupational health of animal shelter employees by live release rate, shelter type, and Euthanasia-related decision. Anthrozoos. 2020;33(1):119–131. doi: 10.1080/08927936.2020.1694316 |
| 70. | Tomasi SE, Fechter-Leggett E, Edwards N, Reddish A, MD C, Nett RJ. Suicide among veterinarians in the United States from 1979 through 2015. J Am Vet Med Assoc. 2019;254(1):104–112. doi: 10.2460/javma.254.1.104.Suicide |
| 71. | Association of Shelter Veterinarians. Position Statement: Well-being of Shelter Veterinarians and Staff. 2022. |
| 72. | Karsten CL, Wagner DC, Kass PH, Hurley KF. An observational study of the relationship between Capacity for Care as an animal shelter management model and cat health, adoption and death in three animal shelters. Vet J. 2017;227:15–22. doi: 10.1016/j.tvjl.2017.08.003 |
| 73. | Crane MF, Phillips JK, Karin E. Trait perfectionism strengthens the negative effects of moral stressors occurring in veterinary practice. Aust Vet J. 2015;93(10):354–360. doi: 10.1111/avj.12366 |
| 74. | Powell L, Reinhard CL, Serpell J, Watson B. A survey of veterinary student and veterinarian perceptions of shelter medicine employment. J Vet Med Educ. 2021. doi: 10.3138/jvme-2021-0112 |
Appendix A: Glossary
Glossary terms
Age Category, Adult – cats and dogs 5 months of age or older
Age Category, Juvenile – cats and dogs under 5 months of age
Age Category, Neonate – cats and dogs 4 weeks of age or younger
Aggregation – gathering animals from different source shelters in one vehicle or location
Analgesia – pain control, usually medication or other therapeutics
Anesthesia – medications that induce unconsciousness and prevent pain
Animals in Care – the number of animals currently housed in the shelter including those housed off-site and in foster homes
Antimicrobial – products such as medications and disinfectants which kill or decrease reproduction of pathogens
Aversive – equipment or practice intended to cause an animal to stop an undesirable behavior by associating it with an unpleasant event
Behavior Assessment – a process of observing and interpreting an individual animal’s behavior throughout their shelter stay, in order to better understand their needs, address welfare concerns, and make appropriate handling, outcome and placement decisions
Behavior Evaluation – a structured procedure or test in which an animal’s responses to a series of sub-tests performed one after the other are observed and interpreted
Capacity for Care – the total resources (e.g. humane housing, trained personnel, medical care, appropriate outcomes) required to promote positive welfare as described by the Five Domains for all the animals in (or coming into) the shelter’s care
Certificate of Veterinary Inspection (CVI) – official document issued by an accredited veterinarian certifying that the animals identified on the document have been inspected and meet the importation criteria of the destination state; also known as a “health certificate”
Circadian Rhythm – internal biological process that regulates the sleep–wake cycle and repeats approximately every 24 hours
Cleaning – removal of dirt, oils, grime, and organic materials; includes both physical cleaning (i.e. scooping feces, scrubbing dirt) and chemical cleaning (i.e. application of a detergent or degreaser)
Co-Housing (Group Housing) – housing more than one animal in the same primary enclosure
Community Cat – all outdoor dwelling cats regardless of socialization status; community cats may be owned, unowned, free-roaming, or feral
Control Pole (i.e. Rabies Pole or Catch Pole) – rigid metal pole with an internal cable that forms an adjustable noose at one end
Deep Cleaning (Full Cleaning) – cleaning followed by sanitation (i.e. application of a disinfectant); used when a cage is heavily soiled, contaminated with infectious pathogens, or a different animal will be occupying the enclosure
Degreasers – strong detergents
Dental Probing – procedure in which a dental instrument called a “probe” is used to identify and measure periodontal pockets around the teeth
Dermatophytosis (Ringworm) – skin disease caused by pathogenic fungal organisms, most commonly Microsporum or Trichophyton species
Destination Shelter – organization that receives relocated animals from a source shelter
Detergent – chemical used during the cleaning process designed to break down oils and suspend particles so they can be removed by wiping or rinsing
Disinfection – inactivation of pathogens, usually through application of a properly diluted chemical product for a specified period of time
Efficacy – capacity for producing the desired outcome; how well something works
Feral Cat – unsocialized “wild” domestic cats living outside without human contact; fearful and avoidant of human interaction much like other wildlife species
Fomite – any object that may become contaminated and contribute to the spread of pathogens (e.g. clothing, equipment, hands)
Footbath – a floor container filled with disinfectant intended to be stepped in to reduce pathogen load on footwear
Forensic Evaluation – gathering and reviewing all crime-related evidence including the forensic physical examination or necropsy, diagnostic test results, reports from others involved in the investigation, documentation such as photographs or videos, and evidence collected from the animal and scene, in order to render an expert opinion about the case
Forensic Physical Examination – comprehensive physical examination, including normal and abnormal findings, that carefully documents health status, identifies abnormalities, and collects evidence
Foster Care – temporary housing in the home of a community member where a shelter-owned animal receives individualized care and monitoring, regular positive social interaction with people, and physical, sensory and mental enrichment
High Consequence Pathogen – contagious disease with the potential to cause significant harm or death, spread rapidly, or infect humans
Humane Investigator – person who investigates animal abuse and neglect, may work for a shelter or a law enforcement agency
Importation – movement of animals into a state or country intended to be their final destination
Incident Command Structure (ICS) – standardized approach to the control and coordination of emergency response providing a common hierarchy within which responders from multiple agencies can be effective
Infectious Dose – number of pathogens required to cause infection
Infrastructure – organizational structures and facilities (e.g. buildings, roads, power, supplies, personnel) needed for the operation of an organization, community, or society
Intact (Entire, Unsterilized) – animal with a complete reproductive tract
Isolation – housing for clinically ill (symptomatic) animals infected with a contagious disease that physically separates them from those who are not infected
Just in Time Training – educational process that provides knowledge and skills at the time they are needed
Length of stay (LOS) – period of time (usually in days) that an animal is in the shelter’s care; calculated as the difference between the date of intake and the date of final outcome; often used as an average or median for species and life stage
Liability – action or omission for which a person or organization can be held legally responsible
Maltreatment – behavior towards a person or animal that involves physical abuse, sexual abuse, emotional abuse, or neglect
Memorandum of Understanding (MOU) – a document describing the broad outlines of an agreement that two or more parties (usually organizations) have reached
Metrics – numerical measures of shelter performance including intakes, returns, euthanasia rates, live outcome rates, lengths of stay (LOS), community services, etc.
Morbidity – number of animals infected by a specific disease in a population
Mortality – number of animals who die due to a specific disease or condition in a population
Multi-Compartment Enclosures – housing with at least two separate areas connected by a door, pass-through, or portal, and allows open access to both sides of the housing except during cleaning or handling
National Incident Management System (NIMS) – guidelines that define operational systems for personnel working together during emergencies; provides communities and organizations with shared vocabulary, goals and processes needed to successfully respond to a disaster or incident
Necropsy – an animal post-mortem examination (autopsy)
Neuter – surgical procedure in which the male reproductive organs (testicles) are removed; occasionally used to indicate surgical sterilization in females
Orthopedic – surgical procedure focused on repair of bones and the skeletal system
Outbreak – increase in the number or severity of cases of a disease in a population; can include but not limited to disease spread inside the shelter
Partner Shelter – in disaster response, a shelter not directly impacted by the emergency but providing any kind of assistance to the impacted shelter or community
Pathogen – biological agent that can cause disease, including bacteria, viruses, protozoa, fungi, and parasites
Pathway Planning – proactive process of determining the most appropriate outcome for each animal, which steps are necessary to achieve that outcome, and reassessment of the pathway as needed
Personal Protective Equipment (PPE) – equipment worn to minimize exposure to hazards that cause workplace injuries and illnesses; also used to minimize transmission of pathogens between animals (e.g. gloves, gowns, goggles, shoe covers)
Personnel – all administration, management, staff and volunteers working at or for an organization, both paid and unpaid
Physical Description – includes species, weight, coat color, markings, sex, neuter status, age, and breed when appropriate
Polishing – procedure in which paste is used to buff and smooth surface defects in teeth caused by scaling or wear
Population Rounds – regular holistic assessment of the shelter population (usually daily) to ensure that each animal has a plan and that all needs and critical points of service are promptly met
Positive Reinforcement – rewarding a desired behavior with a pleasant reward
Practice of Veterinary Medicine – defined by state practice acts and limited to licensed individuals; diagnosis, prognosis, treatment, and prevention of animal disease, illness, pain, deformity, defect, injury, or other physical, dental, or mental conditions by any medical or surgical method
Prophylactic – preventive or presumptive treatment or management of disease before it becomes clinically apparent
Quarantine – housing for healthy animals exposed to and potentially incubating a contagious disease that physically separates them from clinically ill or unexposed animals
Relocation – program or organized effort to transport animals from one sheltering organization (source) to another (destination) locally, regionally, or internationally
Return to Field (Shelter Neuter Return) – outcome process of sterilizing unowned cats and returning them to their home situation after shelter intake
Risk Assessment – a process to identify possible incidents or problems, their likelihood of occurring, and steps that can be taken to control or reduce frequency and/or severity of harm
Sanitation – process of both cleaning and disinfection
Scaling – dental procedure in which tartar or calculus is physically removed from the surfaces of the teeth (manual or ultrasonic)
Shelter – organization of any type or size that provides temporary housing for companion animals; includes foster-based rescues, non-profit humane societies and SPCAs, municipal animal control facilities, and hybrid organizations
Source Shelter – organization that prepares and sends animals for relocation to a destination shelter
Spay – surgical procedure where the female reproductive tract (ovaries and/or uterus) is removed
Spot Cleaning – cleaning process that includes tidying and removal of soiled objects and stains; used when a cage is lightly soiled AND the animal is remaining in the same enclosure; less disruptive than deep cleaning
Sterilization – collective term for surgeries that remove the reproductive organs from dogs and cats with the intent of permanently preventing offspring; also known as spay-neuter, neutering, and de-sexing
Surgical Suite – separate room of the medical department where surgeries are performed
Test, Diagnostic – medical test administered to animals with clinical signs of disease or injury to determine the cause
Test, Screening – medical test administered to determine whether a sub-clinical or inapparent disease, condition or exposure is present
Tethering – using a chain, rope, leash or cord to attach a dog to a stationary object with the intention of restraining them while unattended
Transfer (of Ownership or Custody) – formally handing over possession of an animal to another shelter or individual, typically as a transfer of ownership
Transport – movement of animals from one location to another, including intrastate, interstate, and international transportation
Veterinary Client Patient Relationship (VCPR) – situation in which a veterinarian has assumed case responsibility, has become familiar with the individual animal, population and/or premises, and has consent from the owner or current caretaker to provide treatment and management of diseases or conditions; defined by state practice acts, often required to perform veterinary services
Veterinarian, Licensed – person who holds a current license to practice veterinary medicine in the state in which services are provided
Veterinarian, Shelter – veterinarian with experience and training in the practice of veterinary medicine in animal shelters, may be employed or contracted by a shelter, or consulting
Veterinary Supervision, Direct – licensed veterinarian is readily available on the premises
Veterinary Supervision, Indirect – licensed veterinarian has given either written or oral instructions for management of the patient and is readily available by telephone or other forms of immediate communication, but is not necessarily on the premises
Zoonotic Disease (Zoonoses) – infectious disease spread between animals and people
Abbreviations
DAPP (DHPP/DA2PP): Canine Distemper, Adenovirus type 2 (Hepatitis virus), Parainfluenza Virus, Parvovirus
FVRCP (HCP): Feline Viral Rhinotracheitis (Herpesvirus), Calicivirus, Panleukopenia Virus
ICS: Incident Command System
IN: Intranasal; into the nose
LOS: Length of Stay
MLV: Modified Live Virus; a type of vaccine
MOU: Memorandum of Understanding
NIMS: National Incident Management System
PPE: Personal Protective Equipment
RTF: Return to Field
SPCA: Society for the Prevention of Cruelty to Animals
SQ: Subcutaneous; under the skin
TNR (TNVR): Trap-Neuter-(Vaccinate)-Release
CVI: Certificate of Veterinary Inspection
VCPR: Veterinary Client Patient Relationship
Appendix B. Examples of Core Shelter Protocols
| Management and Record Keeping |
|
| Population management |
|
| Animal Handling |
|
| Facility Design and Animal Housing |
|
| Sanitation |
|
| Medical Health |
|
| Shelter Surgery |
|
| Forensics |
|
| Behavioral Health and Mental Well-being |
|
| Euthanasia |
|
| Animal Transport and Relocation Programs |
|
| Disaster Response |
|
| Public Health |
|
Appendix C. Personal Protective Equipment During Sanitation
| Protective layer Animal population | Gloves | Outer clothing layer (gown, scrubs) | Shoe covers or Dedicated boots |
| Healthy animals | Gloves OR hand hygiene before and after care | Optional | Recommended when entering soiled enclosure |
| Non-contagious medical conditions | Gloves OR hand hygiene before and after care | Optional | Recommended when entering soiled enclosure |
| Mild contagious disease (e.g. typical URI, CIRD) or vulnerable animals | Gloves AND hand hygiene before and after care required | Recommended (change after handling) | Recommended when entering soiled enclosures |
| High consequence contagious disease - diagnosis or known exposure (e.g. Parvo-, Distemper-, or Panleukopenia viruses) |
Gloves AND hand hygiene before and after care required | Required -Disposable gown recommended |
Required when entering isolation area |
| *Adjustments based on individual and population disease risk may be indicated. Change PPE between individual enclosures or wards/areas based on disease risk. | |||
Appendix D. Forensics Resources for Shelters
- Standards and Best Practices
■ Touroo, R., Baucomb, K., Kessler, M, Smith-Blackmore, M. “Minimum standards and best practices for the clinical veterinary forensic examination of the suspected abused animal” in Forensic Science International: Reports, Volume 2, December, 2020.
■ Brownlie, HW Brooks, and R. Munro. “The veterinary forensic necropsy: a review of procedures and protocols.” Veterinary pathology 53.5 (2016): 919-928.
- Books
■ Veterinary Forensic Medicine and Forensic Sciences Eds. Byrd JH, Norris P, Bradley-Siemens, N. CRC Press, 2020.
■ Veterinary Forensic Pathology, Volumes 1&2. Ed. Brooks J, Springer, 2018.
■ Veterinary Forensics: Investigations, Evidence Collecting and Expert Testimony. Eds. Rogers ER, Stern A., CRC Press. 2018.
- Organizations
■ International Veterinary Forensic Science Association (IVFSA). https://www.ivfsa.org
■ American Academy of Forensic Science (AAFS). https://www.aafs.org
■ American College of Veterinary Pathologists (ACVP). https://www.acvp.org
Appendix E: Environmental Management Considering an Animal’s Five Senses
| Sense | Description/Perception | Management |
| Hearing |
|
|
| Smell |
|
|
| Taste |
|
Offer delicious foods to entice, engage, and create positive associations
|
| Sight |
|
|
| Touch |
|
|
|
|
Appendix F: Opportunities for Positive Social Contact in the Shelter
| Type of social contact | References | |
| Calm interactions with people | Quiet time (e.g. time out of enclosure in an office) | Protopopova et al. 2018 |
| Petting, massage | Hennessy 1998 | |
| Shiverdecker et al. 2013 | ||
| Dudley et al. 2015 | ||
| McGowan et al. 2018 | ||
| Perry et al. 2020 | ||
| Reading books | Tuozzi et al. 2021 | |
| Active interactions with people | Play with toys (e.g. fetch, tug) | Coppola et al. 2006 |
| Shiverdecker et al. 2013 | ||
| Hunt et al. 2022 | ||
| Walking, jogging | Braun 2011 | |
| Menor-Campos et al. 2011 | ||
| Training using positive reinforcement | Laule 2003 | |
| Thorn 2006 | ||
| Grant and Warrior 2017 | ||
| Kogan et al. 2017 | ||
| Interactions with members of the same species | Group housing of compatible animals (see Facilities: Co-housing) | |
| Playgroups (dogs) | Belpedio et al. 2010 | |
| Foster Care | Overnight fostering (dogs) | Gunther et al. 2019 |
| Gunter et al. 2021 | ||
Appendix G: Ideas for Enrichment Within Shelter Enclosures
| Type of enrichment | Examples | Additional considerations |
| Feeding | Commercially available or home-made devices that provide mental stimulation by requiring animals to work to extract food such as food puzzle toys, cardboard boxes, or plastic cups (Griffin 2006, 2009a; Schipper 2008; Shepherdson 1993) | Provide to dogs individually because they are competitive eaters; can be given to cats housed singly or in amicable groups (Dantas et al. 2011) |
| Scent | Certain essential oils, food scents, prey odors, and catnip (Ellis and Wells 2010, Graham et al. 2005, Binks et al. 2018, Amaya et al. 2020, Murtagh et al. 2020) | Pheromone products without a comprehensive plan for stress reduction and enrichment are less likely to be effective (Janeczko 2022) |
| Auditory | Classical music, soft rock, reggae, nonmusical white noise, audiobooks, or (for cats) species-specific specially composed music (Kilcullen-Steiner and Mitchell 2001; Wells et al. 2002; Kogan et al. 2012; Snowdon et al. 2015; Bowman et al. 2015, 2017; Brayley and Montrose 2016; Hampton 2020) | Choice of sound type and volume is critical. Reducing excess noise from animal and non-animal sources may be more important than adding additional sound. Balance music preferences of animals and personnel to optimize benefits. |
| Visual | Windows overlooking natural environment | Enrichment videos may be less helpful for cats and dogs compared to other species, as they Dogs and cats do not seem to spend a significant amount of time looking at the screen and lose interest if the videos are played for extended (i.e. multiple hours) periods of time (Graham et al. 2005; Ellis and Wells 2007). |
| Protected outdoor access | ||
| Visual access to members of the same species | ||
| Interesting stimuli such as aquariums or bubbles | ||
| Videos | ||
| Tactile | Soft bedding Scratching posts Petting Massage |
Appendix H: Disaster Response Resources
- Standards and Best Practices:
• NASAAEP Animal Evacuation and Transportation
• NASAEEP Disaster Veterinary Care: Best Practices
• NASAAEP Emergency Animal Decontamination Best Practices
• NASAAEP Emergency Animal Sheltering Best Practices
• NASAAEP Animal Search and Rescue
• FEMA Hazard Mitigation Planning
- Books
• Animals in Disasters, Dick Green, ed. Elsevier. 2019
• Animal Management and Welfare in Natural Disasters, James Sawyer & Gerardo Huertas, eds. Routledge: Taylor Francis Group, 2018
• Veterinary Disaster Response, Wayne E. Wingfield & Sally B. Palmer, eds. Wiley Blackwell, 2009
Appendix I: Example ICS Chart for Animal Shelters
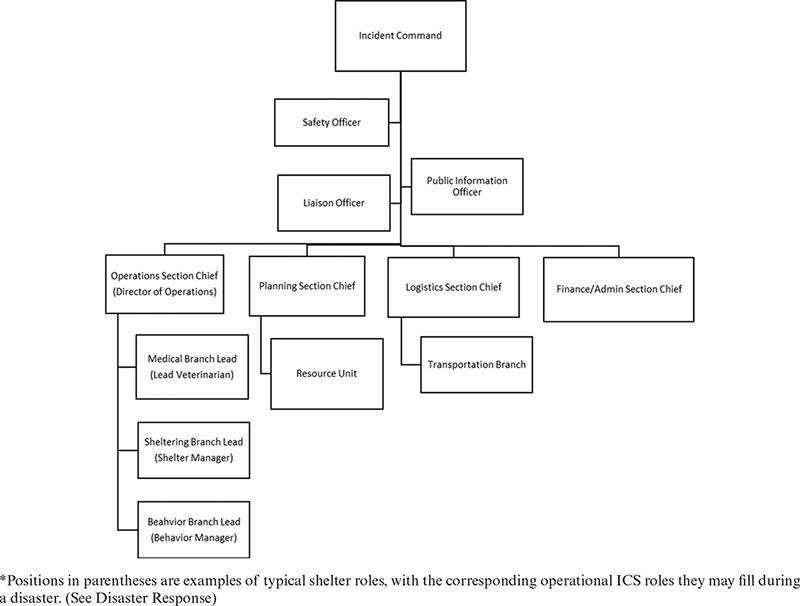
Appendix J: Resources for Workplace Safety
| Organization | Area of concern | Website | |
| CDC | Center for Disease Control and Prevention | -United States Health Protection Agency | http://www.cdc.gov |
| NIOSH | CDC’s National Institute for Occupational Safety and Health | -Workplace Safety Guidance | https://www.cdc.gov/niosh/index.htm |
| OSHA | Occupational Safety and Health Administration | -Occupational Health Regulations | https://www.osha.gov/ |
| EPA | Environmental Protection Agency | -Sanitizers and Disinfectants | https://www.epa.gov/ |
| -Indoor Air Quality | |||
| -Topical Pesticides | |||
| -Wastewater control | |||
| FDA | Food and Drug Administration | -Animal Food Safety | https://www.fda.gov/ |
| -Animal Drugs | |||
| -Medical Devices | |||
| DEA | Drug Enforcement Administration | -Drug Disposal | https://www.dea.gov/ |
| -Controlled Substances | |||
| State Health Departments and Departments of Agriculture | -Reportable Diseases | https://www.cdc.gov/publichealthgateway/healthdirectories/healthdepartments.html https://www.vetca.org/ |
|
| -Animal Bites and Scratches | |||
| -Animal Carcass Disposal | |||