ORIGINAL RESEARCH ARTICLE
Incidence of perioperative hypothermia in a high-quality high-volume spay/neuter setting and association with environmental temperature
Jennifer Rodriguez-Diaz1, Galina Hayes2*, Leslie Appel3, Nicole Buote2 and Michelle Moyal4
1College of Veterinary Medicine, Cornell University, Ithaca, NY, USA; 2Section of Small Animal Surgery, Department of Clinical Studies, College of Veterinary Medicine, Cornell University, Ithaca, NY, USA; 3Shelter Outreach Services, College of Veterinary Medicine, Cornell University, Ithaca, NY, USA; 4Primary Care Surgery Service, Department of Clinical Studies, College of Veterinary Medicine, Cornell University, Ithaca, NY, USA
Abstract
Introduction: Perioperative inadvertent hypothermia (PIH) can prolong anesthetic recovery times. Study goals included determining PIH incidence (rectal temperature<36°C (96.8°F)) in the high-quality high-volume spay/neuter (HQHVSN) setting and evaluating associations between environmental temperature and PIH incidence. Secondary objectives included evaluating associations between PIH incidence, anesthesia recovery times, and postoperative pain.
Methods: Prospective observational cohort study conducted at five HQHVSN shelters enrolling dogs undergoing ovariohysterectomy or castration and cats undergoing ovariohysterectomy. Blankets and electric heating pads were only used routinely in the immediate postoperative period. Regression models were used to evaluate associations between PIH incidence, environmental temperature, and pain data.
Results: One hundred and forty dogs undergoing 65 castrations and 75 ovariohysterectomies, and 161 cats were enrolled. Mean surgical times were 19.6 (8.0) min (canine ovariohysterectomies), 7.2 (3.0) min (canine castrations), and 10.6 (3.6) min (feline ovariohysterectomies). PIH incidence was 22% (95% CI = 17–27). The risk of developing PIH was associated with environmental temperature, with a 22% increase in the odds of experiencing PIH for each degree centigrade decrease in environmental temperature (OR = 1.22, 95% CI = 1.03–1.44, P = 0.02) over a recorded range of 15.6–26.1°C (60.1–79.0°F). In cats, PIH was associated with a prolongation of time to extubation by 3.8 min (95% CI 2.27–5.37, P = 0.01). On average, each degree centigrade reduction in rectal temperature at extubation was associated with an increase in pain scale of 0.51 units (95% CI = 0.06–0.97, P = 0.03) for both species. In dogs, each degree centigrade reduction in rectal temperature at extubation was associated with a reduction in mechanical threshold (indicating increased sensitivity to pain) of 1.40 N (95% CI = 0.44–2.35, P = 0.004).
Conclusion: PIH is common in a spay/neuter population despite rapid surgical times and is associated with prolonged anesthetic recovery in cats and increased postoperative pain in cats and dogs. Raising the environmental temperature in operative and recovery areas may reduce incidence.
Keywords: Peri-operative; hypothermia; cats; dogs; spay; neuter; anesthesia; surgery
Citation: Journal of Shelter Medicine and Community Animal Health 2023, 2: 27 - http://dx.doi.org/10.56771/jsmcah.v2.27
Copyright: © 2023 J. Rodriguez-Diaz et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 10 November 2022; Revised: 16 April 2023; Accepted: 16 April 2023; Published: 16 June 2023
Competing interests and funding: This study was funded by a grant from Maddie’s fund. The authors have no conflicts of interest to declare.
Correspondence: *G. Hayes, 930 Campus Rd, Ithaca, NY 14853. Email: gmh59@cornell.edu
Reviewers: Jeff Norris, Karla Brestle
Perioperative inadvertent hypothermia (PIH) occurs in cats and dogs when core temperatures fall in association with anesthesia and surgery.1,2 This may occur due to radiant, conductive, or evaporative heat loss in conjunction with anesthesia-related vasodilation and loss of normal thermoregulatory mechanisms. Anesthesia-related vasodilation can cause redistribution of heat from the core to periphery resulting in core temperature decreases.1 Several negative secondary effects of PIH in small animals have been identified, including prolonged anesthetic recovery3,4 and prolonged postoperative anorexia.4 In human adult patients undergoing anesthesia and surgery, PIH has additionally been associated with increased blood loss,5 increased surgical site infection rates,6–8 and exacerbation of postoperative discomfort.9 In human infants, PIH has been associated with hypotension, cardiac arrhythmias, and desaturation10 prompting PIH to be made the subject of a number of quality improvement initiatives.11–13
Identification of the negative outcomes associated with PIH has resulted in an emphasis on its prevention in human and veterinary surgical care through both raised environmental temperatures in surgical and induction areas4,12 and active patient warming measures.14 However, patient warming equipment may not represent a financial priority in primary care or shelter practice, and manpower for both temperature monitoring and individualised patient warming measures may be lacking. Maintaining higher environmental temperatures may be challenging to implement when induction and surgical areas are multiuse in nature.
The incidence of PIH has been assessed at 32–35% in dogs and 50–71% in cats in studies assessing veterinary surgical populations at teaching hospitals1,2,4 but is poorly described in the high-quality high-volume spay/neuter (HQHVSN) setting. The incidence may be substantially lower in spay/neuter practice or primary care, where surgical and preoperative preparation times are typically shorter and clip areas smaller. While there have been recent advances in postoperative pain management and anesthetic protocols that provide for rapid recovery times in animals undergoing out-patient procedures,15 there is a paucity of information available on PIH incidence and impact on outcomes in this setting.
The objective of this study was to determine the incidence of PIH (defined as a temperature <36°C (96.8 °F)) in a high volume spay/neuter setting with facilities typical of those seen in primary care practice and to identify any associations between environmental temperature and PIH incidence in this surgical population and setting. Secondary objectives were to assess the relationship between PIH and anesthesia recovery times and to assess the relationship between patient extubation temperature and postoperative pain levels 1 h following extubation.
Methods
Study design and inclusion criteria
This was a prospective observational study conducted at five animal shelters located in upstate New York area (IACUC exemption 50719-04 Cornell University College of Veterinary Medicine). The shelters hosted sterilization services provided by a single local non-profit corporation (Shelter Outreach Services). The sterilization services met or exceeded the guidelines set by the Association of Shelter Veterinarians for HQHVSN programs.16 The sterilization programs were staffed with varying personnel; however, the staffing structure consisted of a shelter veterinarian with a high level of experience in spay/neuter surgery assisted by a licensed veterinary technician. In addition, between two and four volunteer staff were provided by the shelter partner. Sterilisation services were provided to homeless dogs and cats, as well as dogs and cats from local rescue groups, low-income homes, and barn or feral cats. A convenience sample of surgical cases consisting of cat or dog spays and dog castrations was enrolled in summer over 14 days of observation between May 25th and August 25th 2019. Eligibility criteria consisted of presentation for cat spay, dog spay, or dog castration. Feral animals were defined as dogs that required a rabies pole to handle or cats that required the use of a squeeze cage to handle.
Animals presenting for any additional procedures were excluded
Standardized anesthesia protocols were used. Briefly, for dogs, these consisted of premedication with butorphanol 0.2 mg/kg and dexmedetomidine 3–7 mcg/kg (depending on tractability) given IM followed by induction with a ketamine/midazolam mixture given IV at a dose of 10 mg/kg ketamine/0.2 mg/kg midazolam. Dogs were then intubated and anesthesia maintained with isoflurane inhalant in 100% oxygen. Tractable cats received an identical protocol. Feral cats that could only be handled with a squeeze cage received an entirely IM protocol consisting of dexmedetomidine 35 mcg/kg, butorphanol 0.3 mg/kg, and ketamine 5.0 mg/kg IM. Following sedation 100% oxygen was provided. Prophylactic antibiosis was provided with ampicillin. Postoperative analgesia was provided with meloxicam given subcutaneously prior to induction. Animals did not routinely receive any reversal agents. Rescue analgesia was provided as needed at the discretion of the supervising veterinarians.
Thermal care was provided at the discretion of the clinical personnel—available equipment consisted of blankets and electrical heating pads. Electrical heating pads were used with a towel interposed between the pad and the animal. Data collection was performed by a single unblinded individual (JRD) following a written protocol and using an electronic data entry system (RedCap). Dogs were extubated once the swallowing reflex returned. Cats were extubated when they were showing return of jaw tone/ear flick.
Data collection
Animals that satisfied the eligibility criteria (cat spays, dog spays, and castrations) were enrolled consecutively on arrival and followed until 1 h post-extubation. Data collected included species, sex, estimated age, body weight, whether the animal was feral, ASA physical status classification, clip area as an estimated % of total body area, and any thermal care measures provided. Clip area was estimated using the Henrikkson body mapping chart.17 The nature of the surgical procedure and the surgical time was also recorded. Environmental temperatures were measured using mercury thermometers and did not vary between the induction, surgery, and recovery areas.
Rectal temperature was measured at several time points using a calibrated electronic rectal thermometer (Welch Allyn Sure Temp Plus 690/692) with a temperature probe cover. Thermometer calibration was performed using a heat block. Time points for temperature measurement consisted of pre-medication, induction, the start and end of surgery, extubation, and 1 h following extubation. The animal was recorded as experiencing PIH if the rectal temperature fell below 36.0°C (96.8°F) at any time point. This cut-point was selected as within the range of ‘moderate hypothermia’ defined in previous studies1,2 and associated with negative clinical outcomes in other veterinary populations.4 The thermometer measurement range was 26 to 44°C (78.8–111.2°F). The intraoperative rate of temperature decline was calculated as the temperature at the end of surgery subtracted from the temperature at the start of surgery divided by the duration of surgery for each patient.
The time period from the end of surgery until the point at which the patient had recovered a swallowing reflex (dogs) and return of jaw tone/ear flick (cats) and could be safely extubated was recorded. Feral animals were excluded from the analysis of this endpoint, as they were typically extubated early to avoid injury to staff or in some cases not intubated. Descriptive data on the feral animal population were retained.
Global patient postoperative pain was assessed 1 h following extubation using the 10-item, four-domain feline composite pain scale18 in cats (range 0–28) or the short form Glasgow Composite Measure Pain Scale (CMPS-SF)19 in dogs (range 0–24), where temperament allowed. Pain scoring was not performed in feral animals. Systolic blood pressure was measured for pain scale calculation using a 9.7 MHz doppler ultrasound infant-style probe and unit (Model 811-BTSA; Parks Medical Electronics, Las Vegas, NV) except where the animal could not be safely handled. Following pain scoring, peri-incisional pain was assessed using mechanical threshold testing with a force algometer installed with a 2-mm probe head (Prodplus; Topcat Metrology, Little Downham, Cambridgeshire, United Kingdom). The threshold was recorded as the average of three consecutive pressure readings, with the probe applied 1 cm lateral to the surgical incision and the threshold taken as any pain response.
Statistical methods
Continuous data were assessed for normality using the Shapiro-Wilk test and expressed as mean (SD) where normal and median (interquartile range (IQR)) where not normal. IQR was expressed as the 25th percentile to the 75th percentile. Categorical data were expressed as a proportion together with the group numerator (n) with 95% confidence intervals where appropriate. Targeted group comparisons for categorical data were made using the chi-squared tests when cell numbers were>5 or the Fishers exact test when ≤5. Group comparisons for continuous data were made using the t test where data were normal and the Mann–Whitney test when data were not normal. Differences in proportional incidence of PIH between surgical procedure groups were tested using logistic regression with a group indicator variable. The odds ratio for the association between PIH incidence and environmental temperature was calculated using univariable logistic regression. Goodness of fit was assessed using the Hosmer Lemeshow test. Prior to pooled analysis, canine pain score data were re-scaled to place it on the same 0–28 scale as the feline data. Associations between pain score data and rectal temperature at extubation were assessed using linear regression. The effect of species and procedure type were evaluated as a covariable together with their interaction terms. Model assumptions were checked using the Cook-Weisburg test, graphical assessment of normality of residuals, and Lowess plots to assess linearity across the data range. Associations between mechanical threshold data and rectal temperature at extubation were assessed in a similar manner. Statistical significance was set at P < 0.05. All statistical calculations were performed in Stata 16.0 (StataCorp).
Results
Patient and procedural information
Procedures were performed on 161 cats and 140 dogs that were presented to shelters for neutering. The canine surgical procedures consisted of 65 castrations and 75 spays, while all feline procedures were spays. No data from any patient were excluded. Mean (SD) body weight was 2.60 (0.89) kgs for cats and 17.15 (12.79) kgs for dogs. The median (IQR) estimated age was 1.0 year (0.6–1.8) for dogs and 1.0 year (0.3–1.0) for cats. Feral animals made up 8% (11/140) of the dog and 22% (35/161) of the cat populations. ASA 1 status was considered appropriate for 99% (138/140) of dogs and 98% (158/161) of cats. Clip areas consisted of 5% (0.8) of total body area for dog castrations, 9% (0.5) for dog spays, and 9% (0.2) for cat spays. Mean (SD) surgical times were as follows: canine castrations 7.2 (4.3) min, canine spays 19.6 (8.0) min, and feline spays 10.6 (3.6) min.
Thermal care during surgical procedures consisted of one cat receiving an electrical heating pad and a blanket and one dog being placed on an electrical heating pad, both of which occurred after PIH developed. In the immediate postoperative recovery period between the end of surgery until extubation, 76% (123/161) cats and 73% (102/140) dogs received thermal care consisting of some combination of a blanket/electrical heating pad. Following extubation, 27% (43/161) cats and 38% (53/140) dogs received thermal care consisting of blankets. Environmental temperatures fluctuated between 15.6 and 26.1°C (60.1–79.0°F) with a mean/SD of 21.6 (1.7)°C (70.8 (3.1)°F) over the 14 observed days.
Hypothermia incidence
The overall incidence of PIH was 22% (66/301; 95% CI = 17–27). Point estimates of PIH incidence varied among the different surgical procedure groups (dog castrations = 20% (13/65), dog spays = 16% (12/75), cat spays = 26% (41/161), but these between-group differences did not reach statistical significance (P = 0.23). However, the severity of the PIH was marginally greater in cats compared with dogs. In cats, the median lowest recorded temperature in each cat was 36.6 (35.9–36.9)°C (97.9(96.7–98.5)°F) compared with 36.9(36.4–37.5)°C (98.4(97.5–99.5)°F) in dogs (P < 0.001).
The highest risk period for the development of PIH was between the end of surgery and extubation. Sixteen animals (8 cats, 8 dogs) developed PIH by the end of surgery, with 38 more (26 cats, 12 dogs) by the point of extubation and 12 more (7 cats, 5 dogs) by 1 h post-extubation.
The fluctuations in median rectal temperature over the perioperative period for the different species are illustrated in Fig. 1. For 29% of animals (13% cats, 16% dogs; 39/161 cats, 48/140 dogs, 301 animals total), rectal temperatures continued to fall rather than rise following extubation. On average, the rate of decline of body temperature during surgery was more rapid for cats at 0.10 (0.05)°C/min than for dogs at 0.04 (0.06)°C/min. This difference was significant by a two-tailed test for unpaired samples (t 299 = 8.32, P < 0.001).
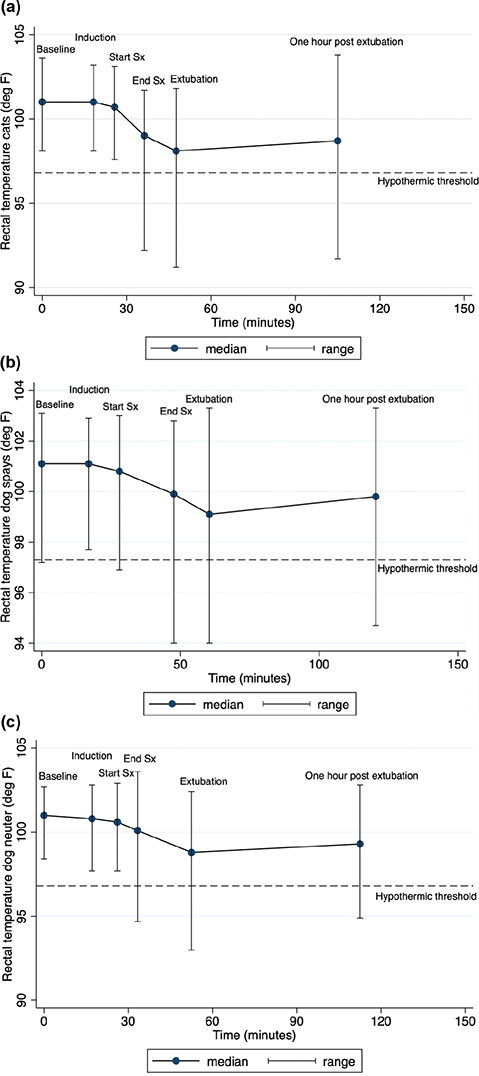
Figure 1. Change in rectal temperatures over the mean surgical phase times of cats undergoing spay and dogs undergoing spay and neuter procedures.
Association between hypothermia incidence and environmental temperature
The overall risk of developing PIH across all surgical groups was associated with environmental temperature, with a 22% increase in the odds of experiencing hypothermia for each degree centigrade decrease in environmental temperature (OR = 1.22, 95% CI = 1.03–1.44, P = 0.02, n = 301). Post-estimation Hosmer Lemeshow P = 0.27 implying appropriate model calibration. In subgroup analysis by species, the association between PIH and environmental temperature was stronger in cats with a 34% increase in likelihood (OR = 1.34, 95% CI = 1.08–1.67, P = 0.01, n = 161) versus dogs (OR = 1.28, 95% CI = 0.95–1.73, P = 0.10). The results of subgroup analysis by species and surgical procedure are shown in Figs. 2 and 3. Each degree centigrade decrease in environmental temperature was associated with a 34% increase in the odds of hypothermia in the cat spay group (OR = 1.34, 95% CI = 1.05–1.63, P = 0.007, n = 161). The strength of the associations fell to 31% and 25% in the dog spay (OR = 1.31, 95% CI = 0.77–1.85, P = 0.21, n = 75) and dog castration groups (OR = 1.35, 95% CI = 0.71–1.79, P = 0.32, n = 65) with loss of statistical significance.
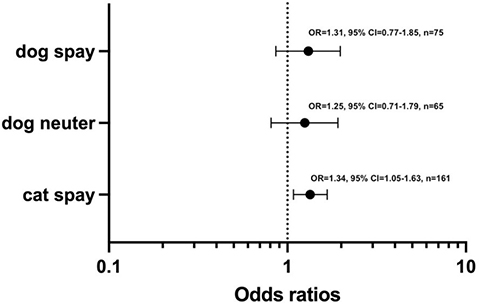
Figure 2. Risk of perioperative inadvertent hypothermia in cats undergoing spay and dogs undergoing spay and neuter procedures per unit centigrade decrease in environmental temperature.
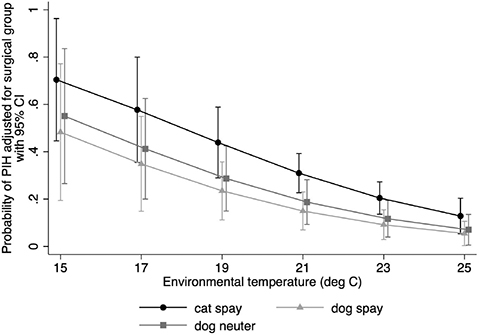
Figure 3. Logistic regression generated predicted probabilities of PIH by surgical procedure group over the range of environmental temperature. Only the association in cats reached statistical significance (OR = 1.34, 95% CI = 1.08–1.67, P = 0.01).
Association between hypothermia and anesthetic recovery time
The mean time period from the end of surgery until extubation in hypothermic cats was 14.2 (8.6) min and in the non-hypothermic cats was 10.4 (7.0) min. This difference was significant by a two-tailed test for unpaired samples (t 124 = -2.55, P = 0.01) and represented a mean prolongation of extubation time of 3.8 min (95% CI = 2.27–5.37) with hypothermia.
A difference was identified in point estimates of mean extubation times between hypothermic and nonhypothermic dogs (15.4 min vs. 14.5 min); however, this difference was not significant by a two-tailed test for unpaired samples (t 127 = -0.38, P = 0.70).
Association between hypothermia and postoperative pain
Pain scores and mechanical thresholds were assessed in 126 cats and 129 dogs. A multivariable linear regression model with pain score as the outcome and rectal temperature at extubation, species, and surgical procedure as predictor variables together with their interaction terms was assessed. Following backward elimination, rectal temperature at extubation and species with no interaction effect were retained in the final model. The loss of the surgical procedure/species interaction term from the model implied that there was no difference in the rectal temperature/pain score association between dog castrations and dog spays, so the results are represented at the species level. The Cook-Weisburg test indicated appropriate homoscedasticity (P = 0.27), residual distribution appeared normal and a Lowess plot suggested linearity. On average, each degree centigrade reduction in rectal temperature at extubation was associated with an increase in pain scale of 0.51 units (95% CI = 0.06–0.97, P = 0.03) for both species. Across the range of extubation temperatures identified, dogs had an average decrease in pain scale units of 2.50 (95% CI = 1.61–3.35, P < 0.001) compared with cats. The linear model predictions for population mean pain score by species are shown in Fig. 4.
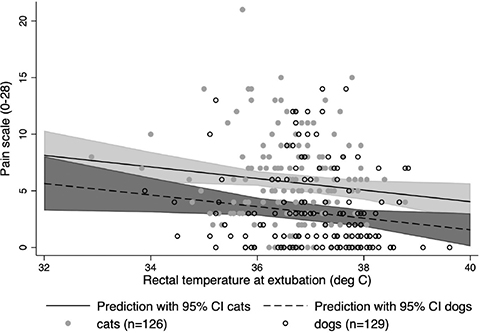
Figure 4. Predicted population averaged pain score means with 95% CI by species with the underlying raw data. Pain score data for dogs was re-scaled to place it on a 0–28 range. The loss of the surgical procedure/ species interaction term from the model implied that there was no difference in the rectal temperature/ pain score association between dog castrations and dog spays, so the results are represented at the species level.
Similar to above, an additional multivariable linear regression model with mechanical threshold as the outcome and rectal temperature at extubation, species, and surgical procedure as predictor variables together with their interaction terms was assessed. Following backward elimination, an association between mechanical threshold and rectal temperature at extubation was identified only in dogs. The Cook-Weisburg test indicated appropriate homoscedasticity (P = 0.37), residual distribution appeared normal, and a Lowess plot suggested linearity. On average, each degree centigrade reduction in rectal temperature at extubation was associated with a reduction in mechanical threshold (indicating increased sensitivity to pain) of 1.40 N (95% CI = 0.44–2.35, P = 0.004). The linear model predictions for the population mean of mechanical threshold in dogs over the range of rectal temperatures at extubation are shown in Fig. 5. The association between extubation temperature and mechanical threshold in cats did not reach statistical significance. Each degree centigrade reduction in rectal temperature at extubation was associated with a reduction in mechanical threshold of 0.16 N (95% CI = -0.15–0.47, P = 0.32).
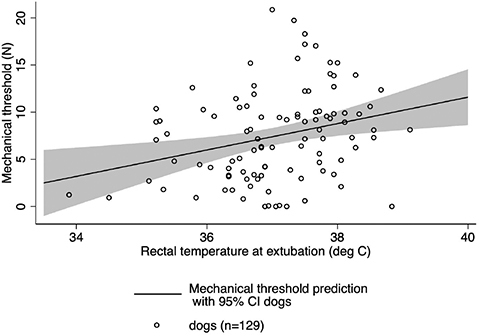
Figure 5. Predicted population averaged mechanical threshold means with 95% CI and underlying raw data in dogs. The association between extubation temperature and mechanical threshold in cats did not reach statistical significance.
Discussion
The results of this study confirmed that PIH is a common complication, affecting approximately one in five animals, even in the context of a spay/neuter surgery population with experienced surgeons and short surgical times. This is similar to the baseline incidence identified in a study investigating human neonatal surgical populations.12 Investigators in that study reduced incidence by half through a collaborative approach led by clinical teams that included measures such as routine temperature monitoring, active patient warming from the point of transport to the anesthesia area and onwards, and raising operating room temperatures to a minimum of 23.3°C (74.0°F). Parallel improvements may be possible in veterinary populations through both the implementation of educational measures directed at informing clinicians about the negative impacts of PIH as well as interventional changes.
Similar to previous studies,4 this study identified an association between environmental temperature and the incidence of PIH. The average temperature observed during this study was 21.6°C (70.9°F), with a 22% increase or decrease in odds of PIH for each degree centigrade fall or rise away from this point. This is unsurprising since the lower the environmental temperature relative to the patient’s core temperature, the steeper the gradient for heat loss. Local active warming measures are unlikely to be able to fully reverse this effect, as access to and exposure of the patient for surgical preparation and procedures are required, preventing the effective creation of a patient ‘heat bubble’ within a cold environment. The optimal room temperature to minimise PIH in the anesthetised patient may not be the room temperature desired by the surgical team to maximize comfort and working efficiency, and temperatures selected typically reflect a balance between these competing demands. Studies conducted on human patient populations investigating the utility of raising environmental temperatures to combat PIH incidence have found temperatures ranging from 23 to 23.9°C (73.4 to 75°F) (contrary to the average 21.6°C (70.9°F) observed in this study) to be effective in reducing PIH incidence in both neonatal and adult populations by 30 to 50%,12,20,21 while a study in cats and dogs found that raising the environmental temperature from 19.9 to 23.9°C (67.8 to 75°F) was associated with a PIH risk reduction of 63%.4
The current study did not attempt to characterize the perceptions of clinical staff regarding PIH or the barriers to prevention. However, the impression of the investigators was that one of the greatest impediments to change was the lack of knowledge regarding the negative effects of PIH on patients coupled with the preference of clinical staff for low environmental temperatures to create a comfortable working environment. For this reason, we strongly suggest that interventional studies in the clinical setting consider staff engagement prior to implementing any environmental modification measures.
As shown in Fig. 1, this study found that core temperature began to fall from the point of induction onwards, with the greatest rate of fall occurring during the surgical procedure, but with the fall persisting between the end of the procedure and extubation. Based on these findings, efforts should be focused on actively warming from the point of induction onwards, if not sooner. In a study focused on re-warming dogs that became hypothermic rather than PIH prevention, 97% of dogs were hypothermic on extubation and 25% of them remained hypothermic 3.5 h later, even after intensive monitoring and warming efforts.22 Similar to a study in human neonates undergoing surgical procedures,10 the majority of dogs became hypothermic following the conclusion of the surgical procedure. This suggests that care providers need to be aware of this as the high-risk period, as monitoring may often be downregulated at this time and active warming measures withdrawn while the patient is moved.
Similar to other studies,4,23 this study found an association between PIH and prolonged recovery times. This association reached statistical significance in cats but not dogs. We suspect that this was due to a greater magnitude of effect in cats due to their average smaller body size, as well as lack of power in the dog group. The association between PIH and delayed recovery is unsurprising given that PIH has been shown to increase anesthetic potency, decrease drug metabolism and reduce cognitive performance.23 It is likely that routine reversal of reversable anesthetic agents (such as dexmedetomidine) would have reduced PIH incidence and severity.
The associations identified between lower patient temperatures at extubation and increased pain levels assessed 1 h later were interesting and are similar to associations identified in human patient populations. Human patients who were hypothermic following total knee arthroplasty had increased postoperative opioid use compared to nonhypothermic patients24 while in another group thermal discomfort was reported as worse than surgical discomfort.25 The increased catecholamine surge that occurs during PIH has been hypothesized to increase pain sensitization.25
This study suffered from several design limitations that limit the conclusions that can be drawn. Animals were recruited over a convenience sample of only 14 days of observation during the summer in five shelters in a single US state. It is possible that incidence of PIH may be different at other times of the year or latitudes, and that the presence of the observer may have altered clinical behaviors over the course of the study. In addition, due to an oversight in the data collection protocol, no site-specific indicator was recorded. This limited the ability to control for site-specific data variation and may have affected the results. Further, perioperative thermal care provided was limited and variable, with no forced air warmers in use. This may have accentuated the impact of environmental temperature on PIH. And finally, although the time from the end of surgery until extubation was selected as a proxy for anesthesia recovery time, monitoring and extubation were performed by a single technician who was multitasking in other areas. This may have resulted in some imprecision in identifying each patient’s ideal extubation point and have contributed to the lack of association found between PIH and recovery times in dogs.
Conclusion
In conclusion, this study identified that PIH is common in high-volume spay/neuter shelter settings with limited manpower and equipment, and that incidence is associated with environmental temperatures. Raising the ambient environmental temperature minimum to around 23°C (73.4°F) may be a simple, low-cost measure to reduce incidence and improve outcomes, including reduced anesthesia recovery times and reduced pain.
Acknowledgement
This study was made possible by a grant from Maddie’s Fund®, #ThanksToMaddie.
References
| 1. | Redondo JL, Suesta P, Serra I, et al. Retrospective study of the prevalence of postanesthetic hypothermia in dogs. Vet Record. 2012;171:374. doi: 10.1136/vr.100476 |
| 2. | Redondo JL, Suesta P, Gil L, et al. Retrospective study of the prevalence of post anesthetic hypothermia in cats. Vet Record. 2012;170:206. doi: 10.1136/vr.100184 |
| 3. | Pottie RG, Dart CM, Perkins NR, Hodgson DR. Effect of hypothermia on recovery from general anesthesia in the dog. Aust Vet J. 2007;85(4):158–162. doi: 10.1111/j.1751-0813.2007.00128.x |
| 4. | Rodriguez-Diaz J, Hayes G, Boesch J et al. Decreased incidence of perioperative inadvertent hypothermia and faster anesthesia recovery with increased environmental temperature: a nonrandomized controlled study. Vet Surg. 2020;49:256–264. doi: 10.1111/vsu.13328 |
| 5. | Schmied H, Kurz A, Sessler DI. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289–292. doi: 10.1016/S0140-6736(96)90466-3 |
| 6. | Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical wound infections and shorten hospitalization. N Engl J Med. 1996;334:1209–1215. doi: 10.1056/NEJM199605093341901 |
| 7. | Seamon MJ, Wobb J, Gaughan J, et al. The effects of intraoperative hypothermia on surgical site infection: an analysis of 524 trauma laparotomies. Ann Surg. 2012;255:789–795. doi: 10.1097/SLA.0b013e31824b7e35 |
| 8. | Walker S, Amin R, Arca M, et al. Effects of intraoperative temperatures on postoperative infections in infants and neonates. J Pediatr Surg. 2020;55:80–85. doi: 10.1016/j.jpedsurg.2019.09.060 |
| 9. | Alfonsi P. Postanesthetic shivering: epidemiology, pathophysiology and approaches to management. Drugs. 2001;61:2193–2205. doi: 10.2165/00003495-200161150-00004 |
| 10. | Morehouse D, Williams L, Lloyd C, et al. Perioperative hypothermia in NICU infants. Adv Neonatal Care. 2014;14:154–164. doi: 10.1097/ANC.0000000000000045 |
| 11. | Cronin JA, Soghier L, Ryan K, et al. A quality initiative for reducing postoperative hypothermia for neonatal intensive care unit surgical patients. Pediatr Qual Saf. 2020;5:318. doi: 10.1097/pq9.0000000000000318 |
| 12. | Brozanski BS, Piazza AJ, Chuo J, et al. STEPP IN: working together to keep infants warm in the perioperative period. Pediatrics 2020;145:2019–2021. doi: 10.1542/peds.2019-1121 |
| 13. | Hanna M, Htun Z, Islam S, et al. A quality improvement initiative to improve perioperative hypothermia rates in the NICU utilizing checklists. Pediatr Qual Saf. 2020;5:367. doi: 10.1097/pq9.0000000000000367 |
| 14. | Franklin MA, Rochat MC, Payton ME, et al. Comparison of three intraoperative patient warming systems. J Am Anim Hosp Assoc. 2012;48:18–24. doi: 10.5326/JAAHA-MS-5650 |
| 15. | Reader R, McCarthy R, Schultz K, et al. Comparison of liposomal bupivacaine and 0.5% bupivacaine hydrochloride for control of postoperative pain in dogs undergoing tibial plateau levelling osteotomy. J Am Vet Med Assoc. 2020;256:1011–1019. doi: 10.2460/javma.256.9.1011 |
| 16. | Griffin B, Bushby PA, McCobb E, et al. The association of shelter veterinarians’ 2016 veterinary medical care guidelines for spay-neuter programs. J Am Vet Med Assoc. 2016;249:165–188. doi: 10.2460/javma.249.2.165 |
| 17. | Henrikkson A, Kuo K, Gerken K, et al. Body mapping chart for estimation of percentage of body surface area in mesocephalic dogs. J Vet Emerg Crit Care. 2022;32:350–355. doi: 10.1111/vec.13173 |
| 18. | Brodani JT, Luna SP, Padovani CR. Refinement and initial validation of a multidimensional composite scale for use in assessing acute postoperative pain in cats. Am J Vet Res. 2011;72:174–183. doi: 10.2460/ajvr.72.2.174 |
| 19. | Reid J, Nolan AM, Hughes JML, et al. Development of the short-form Glasgow composite Measure Pain Scale (CMPS-SF) and derivation of an analgesic intervention score. Anim Welf. 2007;16:97–104. doi: 10.1017/S096272860003178X |
| 20. | Duryea E, Nelson D, Wyckoff M, et al. The impact of ambient operating room temperature on neonatal and maternal hypothermia and associated morbidities: a randomized controlled trial. Am J Obstetr Gynecol. 2016;214:505–560. doi: 10.1016/j.ajog.2016.01.190 |
| 21. | Kim P, Taghon T, Fetzer M, Tobias J. Perioperative hypothermia in the pediatric population: a quality improvement project. Am J Med Qual. 2013;28:400–406. doi: 10.1177/1062860612473350 |
| 22. | Rose N, Kwong G, Pang DS. A clinical audit cycle of postoperative hypothermia in dogs. J Small Anim Prac. 2016;57:447–452. doi: 10.1111/jsap.12547 |
| 23. | Lenhardt R, Marker E, Goll V, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997;87:1318–1323. doi: 10.1097/00000542-199712000-00009 |
| 24. | Benson E, McMillan D, Ong B. et al. The effects of active warming on patient temperature and pain after total knee arthroplasty. Am J Nurs. 2012;112:26–33. doi: 10.1097/01.NAJ.0000414315.41460.bf |
| 25. | Doufas A. Consequences of inadvertent perioperative hypothermia. Best Prac Res Clin Anesthesiol. 2003;17:535–549. doi: 10.1016/S1521-6896(03)00052-1 |