ORIGINAL RESEARCH ARTICLE
Burkholderia-Associated Ocular Disease in Cats
Becky L. Morrow1,2,3* and Diana Ruggiero3
1Department of Biological Sciences, Penn State University, New Kensington, PA; 2College of Veterinary Medicine, Shelter Medicine Distance Education, University of Florida, Gainesville, FL; 3Frankie’s Friends Humane, New Kensington, PA
Abstract
Introduction: Upper respiratory tract disease (URTD) is common in cats, particularly in multi-cat environments such as shelters. The disease can lead to severe ocular pathology and, in some cases, may require enucleation due to endophthalmitis or panophthalmitis.
Methods: Eyes from 16 young cats with URTD that were enucleated because of severe ocular disease (previously ruptured or markedly buphthalmic eyes) were evaluated using Next Generation DNA Sequencing (NGS) with universal bacterial primers to evaluate the entire bacterial population. Eyes were assessed for the presence of feline herpesvirus-1 (FHV-1) and feline calicivirus (FCV) by qPCR and RT-qPCR, respectively.
Results: All eyes had a high relative abundance of Burkholderia, ranging from 43.8 to 98.3%. Burkholderia was the most abundant bacterial genus in 15 of the 16 samples (94%). Of the 16 samples, 8 were positive for FHV-1 (50%), while 1 was positive for FCV (6%).
Conclusion: This study identified an organism that has not been previously associated with severe ocular disease in cats with URTD, and provides a new approach to understanding pathogens by evaluating entire bacterial populations rather than targeting specific microorganisms.
Keywords: upper respiratory tract disease; cats; eye; DNA sequencing; ocular disease; Burkholderia; panophthalmitis; endophthalmitis
Citation: Journal of Shelter Medicine and Community Animal Health 2025, 4: 117 - http://dx.doi.org/10.56771/jsmcah.v4.117
Copyright: © 2025 Becky L. Morrow and Diana Ruggiero. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 11 October 2024; Revised: 23 March 2025; Accepted: 23 March 2025; Published: 19 May 2025
Competing interests and funding: The author declares no potential conflicts of interest.
Correspondence: *Becky L. Morrow, Frankie’s Friends Humane, 730, 5th Avenue, New Kensington, PA, 15068. Email: beckymorrow.dvm@frankies-friends.org
Competing interests and funding: The authors have no conflicts of interest to disclose. Funding for NGS was provided by Frankie’s Friends Humane.
Reviewers: Nick Haley, Aimee Dalrymple
Upper respiratory tract disease (URTD) is common in cats, particularly in multi-cat environments such as shelters. Pathogens most frequently found in cats with URTD are Feline herpesvirus-1 (FHV-1), Feline calicivirus (FCV), Mycoplasma felis (M. felis), Chlamydophila felis (C. felis), and Bordatella bronchiseptica (B. bronchiseptica). Prevalence of infection reaches as high as 59% with FHV-1, 67% with FCV, 24% with C. felis, 84% with M. felis, and 33% with B. bronchiseptica.1–4
Of the two viruses causing URTD, FCV is often dismissed as an ocular pathogen, although it was found in 48% of cats with conjunctivitis, with no accompanying co-infection with M. felis, C. felis, or FHV-1 in over one third of those cats.5 FHV-1 is the most frequent cause of conjunctivitis and is the only primary viral pathogen that causes feline keratitis.6–8 Ulceration of the conjunctiva and cornea secondary to FHV-1 can lead to symblepharon, adhesions between the palpebral conjunctiva and other structures of the eye that can result in reduced vision.9 There is also evidence that FHV-1 is associated with anterior uveitis,10 which can result in synechiae, adhesions of the iris to the cornea or lens, secondary glaucoma, and a non-visual, shrunken globe referred to as phthisis bulbi.11,12
While FHV-1 can cause extensive corneal ulceration and stromal keratitis, secondary bacterial infections are thought to cause deeper ulcers. The most common secondary bacterial pathogens cultured from eyes of cats with conjunctivitis and keratitis are from the genera Streptococcus, Staphylococcus, Pseudomonas, Pasteurella, Enterobacter, and Escherichia.13–15 C. felis has long been considered the primary bacterial pathogen causing feline ocular disease, found in as many as 60% of cats with conjunctivitis.16 Hartmann et al. found nearly equal prevalence of Mycoplasma sp. (49%) and C. felis (56%), with only 27% prevalence of FHV-1.14 Another study showed M. felis to be the most prevalent organism associated with conjunctivitis (9.6%), followed by FHV-1 (6.7%) and C. felis (3.2%).17 Mycoplasma sp. was also found more commonly in cats with conjunctivitis as compared to those who had no history of conjunctivitis, and cats with Mycoplasma sp. were not co-infected with FHV-1. This provides support for Mycoplasma sp. acting as a primary bacterial pathogen.
Infectious keratitis is characterised by a defect of the corneal epithelium with inflammation of the underlying corneal stroma following invasion by pathogens.18 This infection can lead to rapid destruction of the cornea, with stromal malacia and progressive deepening of ulcers leading to perforation and rupture of the globe.19,20 Infectious endophthalmitis results if pathogens invade the anterior or posterior chamber of the eye. This can be due to an exogenous or an endogenous source (hematogenous spread from a primary source). Infectious endophthalmitis may present with concurrent blepharospasm, conjunctivitis, corneal perforation, periocular swelling, third eyelid elevation, ocular discharge, keratitis, uveitis, and chorioretinitis.21 If endophthalmitis occurs along with involvement of the sclera and surrounding extraocular tissues, the term panophthalmitis is more appropriate. Clinical signs of panophthalmitis can include chemosis, proptosis, and limited ocular movement. If scleral involvement is substantial, thinning and perforation may occur, resulting in rupture of the eye.19 With or without corneal or scleral rupture, enucleation is often necessary because of the severity of the damage.
While little information regarding the prevalence of infectious keratitis, endophthalmitis, and panophthalmitis with globe rupture in cats is available, the author routinely sees young cats with acutely ruptured globes and active infections as well as cats with phthisis bulbi or other evidence of previous rupture. Many times, even with the use of topical and systemic antibiotics and antivirals, ocular infections are poorly responsive and the eyes rupture despite treatment. The purpose of this study was to use molecular methods to identify potential pathogens in eyes that were enucleated due to severe URTD-associated ocular disease to ultimately allow for more effective diagnosis and treatment of patients.
Methods
A total of 16 cats from southwestern Pennsylvania that presented to a non-profit veterinary clinic between June 2017 to May 2018 with severe URTD-associated ocular disease were included in this study. The clinic, located in the greater Pittsburgh area, predominantly provided spay/neuter services to cats from the community and regional animal welfare organisations, with a caseload of over 7,000 cats annually. Vaccination, medical treatment, and other surgical procedures, including enucleations, were performed in addition to sterilisation. All cats that needed enucleation in this time period due to URTD-associated ocular disease were included in the study. Only cats with obvious non-infectious causes of ocular disease (i.e. trauma, tumours) were excluded.
All cats had URTD, based on physical examination by the veterinarian (first author), as well as endophthalmitis or panophthalmitis that necessitated enucleation. The majority presented with previously ruptured globes, but three globes were buphthalmic (Fig. 1). The cats were all from different households or animal rescues, and were between the ages of 6 weeks and 7 months of age. Some of the cats were treated with various topical and systemic antibiotics prescribed by other veterinarians prior to presentation for surgery. No antiviral medications were utilised. All cats were negative for Feline Leukemia Virus (FeLV) and Feline Immunodeficiency Virus (FIV). Other than clinical signs of URTD, no other abnormalities were noted on physical examination for any of the cats.
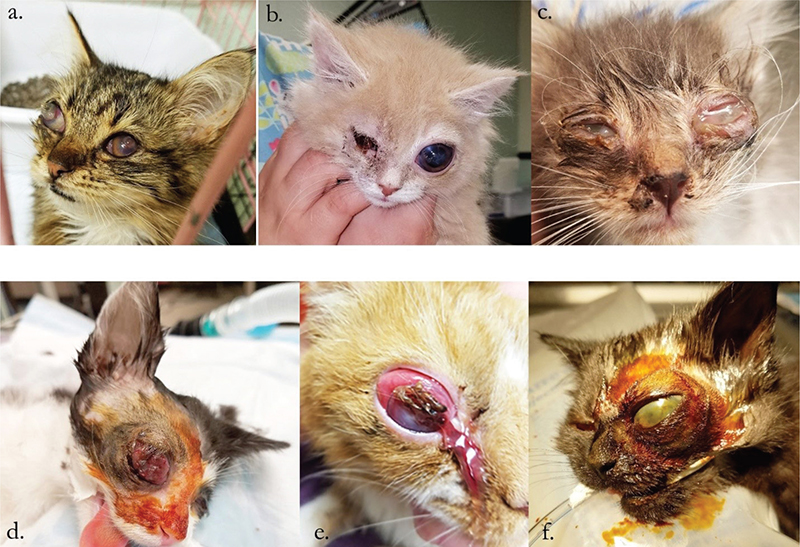
Fig. 1. Six cats with endophthalmitis or panophthalmitis prior to enucleation of the eye(s). Cats in panels a, c, d, and e demonstrate globe rupture while cats in panels b and f show intact, but severely buphthalmic globes.
A combination of butorphanol (0.2 mg/kg), dexmedetomidine (0.01 mg/kg), and ketamine (4.5 mg/mL) was administered intramuscularly. After intubation, cats were maintained at a surgical plane of anaesthesia with 1.5 to 2.0% isoflurane. The skin surrounding the eye being enucleated was shaved and the area (skin and eye) was routinely prepped with 2.0% chlorhexidine scrub and rinsed with isopropyl alcohol. Aseptic technique was used to perform a routine transconjunctival enucleation (by the first author), without rupturing or re-rupturing the globe. The eye was kept on the sterile drape during closure and was subsequently transferred to a sterile container. The enucleated tissue was stored at -20°C for up to 6 months prior to nucleic acid extraction. All cats from this study received one dose of procaine penicillin G (50,000 units/kg) administered at the time of surgery and kittens with evidence of URTD characterised by mucopurulent nasal or ocular discharge were treated with a course of doxycycline (10 mg/kg PO q24h) or amoxicillin with clavulanic acid (12.5 mg/kg, PO q12h) for 10 days.
Processing began by thawing the enucleated eyes. Removal of tissue and aqueous humour from the enucleated eyes was performed using aseptic technique. DNA and RNA were extracted from the samples and positive controls using EasyPrep™ DNA/RNA Miniprep Kit (Bioland Scientific), RNeasy® Mini Kit (Qiagen), and the silica-based method previously described by Boom et al.22 Quantification and assessment of DNA purity was performed with the NanoDrop Lite Spectrophotometer (Thermo Scientific), followed by normalisation to a concentration of 15 ng/μL. Real time reverse transcriptase PCR (qRT-PCR) for FCV was performed using Genesig® Feline Calicivirus Kit and qPCR for FHV-1 with Genesig® Feline Herpesvirus Kit (Primerdesign™ Ltd) and AzuraQuant™ Green Fast qPCR Mix LoRox following manufacturer’s instructions, and with the use of positive controls with viral nucleic acid and negative controls with no nucleic acid template. Internal controls were also used to ensure that negative results were not because of degradation of the nucleic acids. Next Generation Sequencing (NGS) with universal bacterial primers, corresponding to highly conserved regions of the 16S rRNA gene, was performed at Wright Labs (Huntington, PA) using the Illumina MiSeq platform. This resulted in amplification of 253bp fragments of DNA from all bacterial species present in the samples. Sequences were analysed through the Linux based Howard Hughes Medical Institute (HHMI) compute cluster at Juniata College using QIIME, with quality filtering and assignment of Operational Taxonomic Units (OTUs). From the OTUs, bacterial taxa were identified to the genus level via the UCLUST algorithm and Greengenes v. 13.8 database. Relative abundance, the percentage of each bacterial genus present in a particular sample, was also established.
Next Generation Sequencing data collected from cats for previous studies (unpublished data) or diagnostic purposes were evaluated to act as a control and provide additional context for interpretation of the results of this study. Conjunctival swabs were obtained from 35 community cats that were brought in by a rescue group during the same time period that the enucleated eyes were collected. The cats were found in the same geographical area and, similar to the cats that required enucleation, ranged in age from approximately 6 weeks of age to 4 months of age. While 25 of the kittens had evidence of URTD and ocular disease, 10 had no signs of URTD (Table 3). All were negative for FeLV and FIV. Samples were stored at -20°C for approximately 1 month prior to DNA extraction. NGS was performed on the DNA combined from swabs of both eyes. Swabs of the oral cavity or extracted teeth from18 cats ranging from 6 months to 6 years of age, with and without dental disease (Table 4), were collected 2 years after the data from the enucleated eyes were produced. All cats were negative for FeLV and FIV, and did not demonstrate any clinical signs of URTD. The swabs and extracted teeth were stored at -20°C for approximately 1 year prior to processing. A third NGS data set was produced from a study using samples from 45 deceased cats that were either found frozen or died shortly after removal from a large-scale hoarding case. Tissue was collected from the lungs, heart, liver, spleen, and kidneys of deceased cats. These samples had been in storage at -20°C for approximately 10 years as part of the specimen bank maintained by the first author. DNA from each of the 5 organs was pooled after normalisation prior to sending for NGS. Other laboratory procedures and data analysis were the same as described earlier for all three data sets. Because the study utilised discarded tissues or teeth and NGS data from swabs taken for diagnostic purposes, Institutional Animal Care and Use Committee approval was not necessary.
Results
The cats, ranging in age from 6 weeks to 7 months, were typical of the patients seen with URTD in previous and subsequent years. There was no relationship between the 16 cats undergoing enucleation for the severe ocular disease. All 16 samples had a high relative abundance of Burkholderia, ranging from 43.8 to 98.3% (Table 1). Burkholderia was the most abundant bacterial genus in 15 of the 16 samples (94%), with a higher relative abundance of Mycoplasma (55.1%) in the sample with 43.8% Burkholderia (Fig. 2). Mycoplasma was found in seven additional samples in very low amounts, ranging from 0.003 to 0.331% of the total bacterial population. Chlamydophila was found in two samples (0.003–0.030%), Pseudomonas in 15 samples (0.008–1.37%), Streptococcus in 5 samples (0.001–0.076%), and Staphylococcus in 15 samples (0.003–2.52%), all in very low relative abundance (Table 2). Of the 16 enucleated eyes, 8 tested positive for FHV-1 (50%), 1 tested positive for FCV (6%), and 7 tested negative for both FHV-1 and FCV (44%).
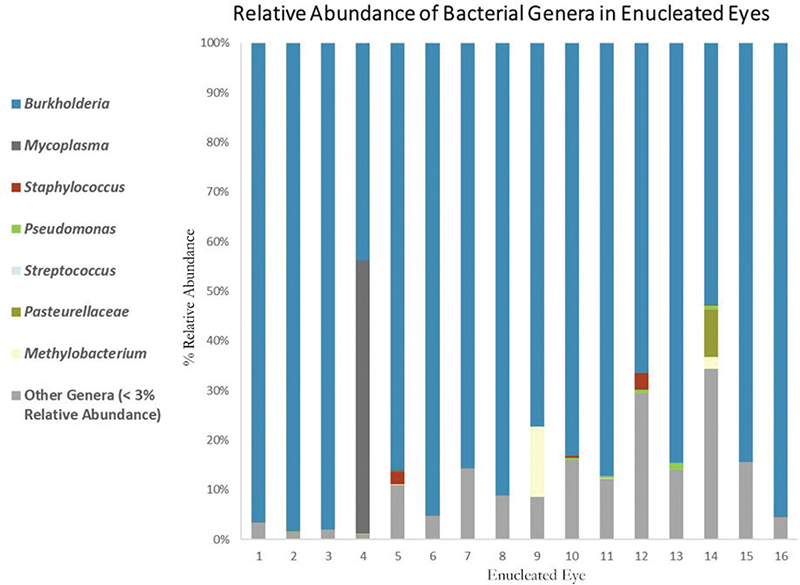
Fig. 2. Relative abundance of bacterial species. Burkholderia was the dominant genus in the enucleated eyes.
Control data provided from the previous analysis of conjunctival swabs taken from cats over the same time period showed that 33 of those cats had either no or extremely low (< 1% relative abundance) levels of Burkholderia sp., while the remaining 2 had relative abundances of 4.1 and 1.5% (Table 3). While these 2 cats had URTD including unilateral mucopurulent ocular discharge, there was no correlation with abundance of Burkholderia sp. and presence of or severity of URTD. NGS data provided from the previous analysis of oral cavity swabs showed 14 out of the 18 cats also had either no or extremely low (< 1% relative abundance) levels of Burkholderia sp. with the remaining cats showing relative abundances of 7% or less (Table 4). Again, there was no correlation between the presence of Burkholderia sp. and the presence of dental disease. The internal organ tissue sample control data set, on the other hand, showed Burkholderia sp. as the most abundant bacterial organism present in 5 of the 45 cats with relative abundances of 85, 79, 57, 25, and 19% (Table 5).
Discussion
Based on the sequence analysis, Burkholderia was the dominant genus, found at a high relative abundance in all enucleated eyes. Burkholderia was first described as a genus in 1992, consisting of seven species that had previously been categorised as Pseudomonas.23 These gram negative, rod-shaped, obligate aerobes are found in a wide range of environmental niches and number well over 100 species.24,25 Closely related Burkholderia species, referred to as the Burkholderia cepacia complex (Bcc), are opportunistic pathogens that can cause severe infections in cystic fibrosis (CF) and immunocompromised patients.26 Two species, Burkholderia mallei (B. mallei) and Burkholderia pseudomallei (B. pseudomallei), are primary pathogens of humans and non-human animals.
Because FHV-1, FCV, C. felis, and M. felis are the most common pathogens associated with feline eye infections and URTD, it was expected that one or more of these pathogens would be found in the infected eyes. None of these pathogens, however, were found in all the tissue samples (Fig. 2), and no other bacterial species was common to all the samples. This suggests that co-infection with FHV-1, FCV, C. felis, M. felis, or another bacterial species is not necessary for development of endophthalmitis or panophthalmitis, and that Burkholderia is the primary pathogen. The high relative abundance of Burkholderia sp. in all the samples, with several approaching 100% (the equivalent of a pure culture in traditional microbiology), further supports Burkholderia as a cause of the eye pathology.
Endophthalmitis can occur when bacteria enter the eye via intraocular surgery, penetrating injury, after severe keratitis with corneal perforation, or via hematogenous spread from a remote primary site.27 The cats included in this study had no signs of ocular trauma and had no history of ocular surgery. While most of the eyes had previously ruptured, the three buphthalmic eyes had intact corneas with no evidence of previous perforation, suggesting an endogenous source of infection rather than entry from outside the eye. The high relative abundance of Burkholderia sp. in the internal organs of five different cats from the control data set (85, 79, 57, 25, and 19%), demonstrates the infection of internal organs as well as the potential for an endogenous source of this bacteria. The lack of Burkholderia sp. found in conjunctival/corneal cultures or by NGS in other studies,28 and very low levels in the control data set from conjunctival swabs in this study, further support endogenous rather than exogenous infection of the eyes.
Very few cases of cats with Burkholderia infections have been described. To the author’s knowledge, this is the first report of Burkholderia-associated endophthalmitis or panophthalmitis in the United States. Two cats from Australia have been reported with primary ocular melioidosis (caused by B. pseudomallei), characterised by acute onset of a ‘red eye’, blepharospasm, and progression to an enlarged, painful, firm globe, followed by loss of vision.29,30 Histopathology on the enucleated tissue from those two cats showed pyogranulomatous uveitis and panophthalmitis with extensive destruction of intraocular structure, as well as moderate numbers of gram-negative bacilli on cytology of the ocular fluid.29 There was no known injury or evidence of primary focus of the infection in those cats. B. pseudomallei endophthalmitis was also reported in a human patient, where there was no history of eye injury but were signs of systemic illness and documented bacteremia.31 Several cases of B. pseudomallei in humans have been described where trauma to the affected eye resulted in endophthalmitis characterised by severe corneal ulceration, chemosis, mucopurulent discharge, and hypopyon.32
Burkholderia cepacia complex (Bcc) has also been documented in cats, but not in association with ocular signs. Five cats with Bcc showed signs of lethargy, inappetence, and non-healing wounds with purulent discharge after contact with a contaminated bottle of chlorhexidine surgical scrub.33 Two of the three cats were euthanised due to severe pyogranulomatous cellulitis and were found to have fibrin thromboses in multiple organs secondary to septicaemia. In humans, Bcc is best known for its opportunistic infections in CF and chronic granulomatous disease patients, and those arising from exposure to contaminated pharmaceuticals, disinfectants, and medical devices.34 Bcc contaminated pharmaceuticals have been well documented as the cause of post-operative endophthalmitis in humans, as well as some cases of keratitis.35
Although a few species can cause severe disease, most species of Burkholderia are not pathogenic for healthy individuals.36 Even after exposure to B. pseudomallei and B. mallei, the two species associated with severe, life-threatening infections, many people were asymptomatic even when they were found to be seropositive.37 Disease due to infection with B. pseudomallei has also occurred many years after exposure.37 This suggests that complex pathogen-host-environment interactions are at play. The Burkholderia genus is composed of a rapidly increasing number of recognised species with a great diversity in specific environmental niches, interactions with other microbes and plants, and antimicrobial resistance mechanisms.38
The pathogenesis or clinical signs produced from the infection may also depend on the route of infection. B. mallei, for example, is the cause of glanders and can cause URTD, ulcerations of the airways, and pneumonia if the organism is inhaled, or pustular skin lesions, multiple abscesses, and sepsis after percutaneous inoculation.36 B. pseudomallei can present with skin lesion, nasal lesions, pneumonia, and clinical signs arising from organs infected via hematogenous spread.37 As was mentioned earlier, B. pseudomallei has been found in cats with signs of systemic illness and endophthalmitis, presumably from hematogenous spread. Even though barriers such as the blood-ocular barrier and blood-brain barrier exist, pathogens can gain entry when bacteria escape the phagocytic machinery and survive within leukocytes.39 This can be another reason for host differences in pathogenesis since genetic differences will affect the ability of the leukocyte to act as a ‘shuttle’.
Improved clinical surveillance and laboratory diagnosis are needed to understand the significance of Burkholderia spp. and the association with severe ocular disease in cats. Burkholderia spp. are not fast-growing organisms and do not always grow in culture. This may be why Burkholderia has not been identified in cats with ocular disease in culture-based studies. Even if a species of Burkholderia can grow in culture, the other bacterial species most commonly isolated from the eyes of cats with conjunctivitis and keratitis grow more rapidly and can easily outcompete Burkholderia. If a species of Burkholderia is, in fact, able to grow on culture, there is still an issue with proper identification since this genus can alter their metabolic and biochemical profile, making phenotypic methods of identification incorrect.40
The use of molecular identification methods will prevent misidentification and demonstrate the presence of bacteria in a sample regardless of the ability to grow in culture. Traditional and real-time PCR (qPCR) have been used in many studies, but mostly use primers that are specific to the organism of interest. NGS and other sequencing-based assays can amplify all bacteria using universal bacterial primers, then determine what species are present by DNA sequencing. NGS can give insight into entire bacterial populations and the relative abundance of the various species present in health and disease.
Limitations of the study
Because all the samples were collected from cats in the same geographical location, further studies from other regions are needed to determine if these findings can be replicated. This can help establish whether these findings have broader applicability and can provide more information on the relationship between Burkholderia and severe ocular disease in cats. Although this study includes control data demonstrating high relative abundances of Burkholderia sp. in internal organs of cats, it does not provide evidence for hematogenous spread of the bacteria to the eyes. Also, because this study utilised frozen tissue, culture and antimicrobial sensitivity was not able to be performed, and the fragments of DNA produced by the NGS were not large enough to discriminate between Burkholderia species. Although these data are lacking, it is reassuring that there were no post-surgical complications, even in patients that were not likely to have ideal immune system function, and that enucleation appears to be curative. While it is uncertain if antibiotic treatment was necessary in addition to enucleation, it is worth noting that the antibiotics utilised in the cats (doxycycline, penicillin and amoxicillin with clavulanic acid) are commonly used to treat B. pseudomallei infections in humans.41 Further studies to determine the species and antibiotic sensitivity will provide stronger evidence that can be used to inform clinical practice guidelines and appropriate use of antimicrobials.
Conclusions
The purpose of this study was to use molecular methods to identify potential pathogens in eyes that were enucleated due to severe URTD-associated ocular disease. Burkholderia, an organism that has not been previously associated with severe ocular disease in cats with URTD, was found in high relative abundance in all the enucleated eyes. While our future goal is to identify the species of Burkholderia and determine antibiotic sensitivity, it is our hope that this study will be a first step to understand the significance of Burkholderia to the feline population and will encourage the use of NGS within the veterinary industry to identify bacterial pathogens that may evade detection by traditional methods.
Author contributions
Becky L. Morrow – Conceptualisation, Formal analysis, Investigation Methodology, Writing original draft.
Diana Ruggiero – Data curation, Project administration (laboratory work), Review and editing.
Acknowledgment/s
Dr. Julie Levy provided assistance with editing.
Dr. Lawerance Bagley provided assistance with editing.
Frankie’s Friends Humane for provision of the samples.
Author notes
Initial data were presented via poster at the Animal Care Expo in 2018.
References
| 1. | Bannasch MJ, Foley JE. Epidemiologic evaluation of multiple respiratory pathogens in cats in animal shelters. J Feline Med Surg. 2005;7:109–119. doi: 10.1016/j.jfms.2004.07.004. |
| 2. | Helps CR, Lait P, Damhuis A, et al. Factors associated with upper respiratory tract disease caused by feline herpesvirus, feline calicivirus, Chlamydophila felis and Bordetella bronchiseptica in cats: experience from 218 European catteries. Vet Rec. 2005;156:669–673. doi: 10.1136/vr.156.21.669. |
| 3. | McManus CM, Levy JK, Andersen LA, et al. Prevalence of upper respiratory pathogens in four management models for unowned cats in the Southeast United States. Vet J. 2014;201:196–201. doi: 10.1016/j.tvjl.2014.05.015. |
| 4. | Fernandez M, Manzanilla EG, Lloret A, et al. Prevalence of feline herpesvirus-1, feline calicivirus, Chlamydophila felis and Mycoplasma felis DNA and associated risk factors in cats in Spain with upper respiratory tract disease, conjunctivitis and/or gingivostomatitis. J Feline Med Surg. 2017;19:461–469. doi: 10.1177/1098612X16634387. |
| 5. | Gerriets W, Joy N, Huebner-Guthardt J, Eule JC. Feline calicivirus: a neglected cause of feline ocular surface infections? Vet Ophthalmol. 2012;15(3):172–179. doi: 10.1111/j.1463-5224.2011.00957.x. |
| 6. | Adam S, Crispin S. Differential diagnosis of keratitis in cats. In Practice. 1995;17(8):355–363. doi: 10.1136/inpract.17.8.355. |
| 7. | Andrew SE. Ocular manifestations of feline herpesvirus. J Feline Med Surg. 2001;3(1):9–16. doi: 10.1053/jfms.2001.0110. |
| 8. | Stiles J. Ocular manifestations of feline viral diseases. Vet J. 2014;201(2):166–173. doi: 10.1016/j.tvjl.2013.11.018. |
| 9. | Nasisse MP. Manifestations, diagnosis, and treatment of ocular herpesvirus infection in the cat. Compend Contin Educ Small Anim Pract. 1982;4:962–970. |
| 10. | Maggs DJ, Lappin MR, Reif JS, et al. Evaluation of serologic and viral detection methods for diagnosing feline herpesvirus-1 infection in cats with acute respiratory tract or chronic ocular disease. J Am Vet Med Assoc. 1999;214:502–507. doi: 10.2460/javma.1999.214.04.502. |
| 11. | Gould D. Feline Herpesvirus-1: ocular manifestations, diagnosis and treatment options. J Feline Med Surg. 2011;13(5):333–346. doi: 10.1016/j.jfms.2011.03.010. |
| 12. | Labelle P. The eye. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. Elsevier; 2017, pp. 1265–1318. |
| 13. | Ion L, Ionascu I, Birtoiu A. Melting keratitis in dogs and cats. Agric Agric Sci Proc. 2015;6:342–349. doi: 10.1016/j.aaspro.2015.08.090. |
| 14. | Hartmann AD, Hawley J, Werckenthin C, Lappin MR, Hartmann K. Detection of bacterial and viral organisms from the conjunctiva of cats with conjunctivitis and upper respiratory tract disease. J Feline Med Surg. 2010;12(10):775–782. doi: 10.1016/j.jfms.2010.06.001. |
| 15. | Suter A, Voelter K, Hartnack S, Spiess BM, Pot SA. Septic keratitis in dogs, cats, and horses in switzerland: associated bacteria and antibiotic susceptibility. Vet Ophthalmol. 2018;21(1):66–75. doi: 10.1111/vop.12480. |
| 16. | Gerhardt N, Schulz BS, Werckenthin C, Hartmann K. Pharmacokinetics of enrofloxacin and its efficacy in comparison with doxycycline in the treatment of Chlamydophila felis infection in cats with conjunctivitis. Vet Rec. 2006;159(18):591–594. doi: 10.1136/vr.159.18.591. |
| 17. | Low HC, Powell CC, Veir JK, Hawley JR, Lappin MR. Prevalence of feline herpesvirus 1, Chlamydophila felis, and mycoplasma spp DNA in conjunctival cells collected from cats with and without conjunctivitis. Am J Vet Res. 2007;68(6):643–648. doi: 10.2460/ajvr.68.6.643. |
| 18. | Thomas PA, Geraldine P. Infectious keratitis. Curr Opin Infect Dis. 2007;20(2):129. doi: 10.1097/QCO.0b013e328017f878. |
| 19. | Leal SM Jr, Rodino KG, Fowler WC, Gilligan PH. Practical guidance for clinical microbiology laboratories: diagnosis of ocular infections. Clin Microbiol Rev. 2021;34(3):e0007019. doi: 10.1128/CMR.00070-19. |
| 20. | Greene CE. Infectious Diseases of the Dog and Cat. 4th ed. St. Louis, MO: Elsevier/Saunders; 2012, p. 1063. |
| 21. | Esson DW. Clinical Atlas of Canine and Feline Ophthalmic Disease. Hoboken, NJ: John Wiley & Sons; 2015, pp. 280–281. |
| 22. | Boom RCJA, Sol CJ, Salimans MMM, et al. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. |
| 23. | Yabuuchi E, Kosako Y, Oyaizu H, et al. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus pseudomonas homology Group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. Nov. Microbiol Immunol. 1992;36(12):1251–1275. doi: 10.1111/j.1348-0421.1992.tb02129.x. |
| 24. | Parte AC, Sardà Carbasse J, Meier-Kolthoff JP, Reimer LC, Göker M. List of prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. Int J Syst Evol Microbiol. 2020;70:5607–5612. doi: 10.1099/ijsem.0.004332. |
| 25. | Woods DE, Sokol PA. The genus Burkholderia. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E, eds. The Prokaryotes. 3rd ed. New York, NY: Springer; 2006, pp. 848–860. |
| 26. | Eberl L, Vandamme P. Members of the genus Burkholderia: good and bad guys. F1000Research. 2016;5:1007. doi: 10.12688/f1000research.8221.1. |
| 27. | Callegan MC, Engelbert M, Parke DW, Jett BD, Gilmore MS. Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin Microbiol Rev. 2002;15(1):111–124. doi: 10.1128/CMR.15.1.111-124.2002. |
| 28. | Leis ML. An update on the ocular surface bacterial microbiota in small animals. Vet Clin Small Anim Pract. 2023;53(2):299–318. doi: 10.1016/j.cvsm.2022.10.004. |
| 29. | Parkes HM, Shilton CM, Jerrett IV, et al. Primary ocular melioidosis due to a single genotype of Burkholderia pseudomallei in two cats from Arnhem Land in the Northern Territory of Australia. J Feline Med Surg. 2009;11(10):856–863. doi: 10.1016/j.jfms.2009.02.009. |
| 30. | Dance DA. Melioidosis: the tip of the iceberg? Clin Microbiol Rev. 1991;4(1):52–60. doi: 10.1128/CMR.4.1.52. |
| 31. | Chen KJ, Sun MH, Hou CH, Sun CC, Chen TL. Burkholderia pseudomallei endophthalmitis. J Clin Microbiol. 2007;45(12):4073–4074. doi: 10.1128/JCM.01467-07. |
| 32. | Siripanthong S, Teerapantuwat S, Prugsanusak W, et al. Corneal ulcer caused by Pseudomonas pseudomallei: report of three cases. Rev Infect Dis. 1991;13:335–337. doi: 10.1093/clinids/13.2.335. |
| 33. | Wong JK, Chambers LC, Elsmo EJ, et al. Cellulitis caused by the Burkholderia cepacia complex associated with contaminated chlorhexidine 2% scrub in five domestic cats. J Vet Diagn Invest. 2018;30(5):763–769. doi: 10.1177/1040638718782333. |
| 34. | Mahenthiralingam E, Urban T, Goldberg J. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol. 2005;3:144–156. doi: 10.1038/nrmicro1085. |
| 35. | Ho MC, Kang EYC, Yeh LK, et al. Clinico-microbiological profile of Burkholderia cepacia keratitis: a case series. Ann Clin Microbiol Antimicrob. 2021;20:6. doi: 10.1186/s12941-020-00407-6. |
| 36. | Coenye T, LiPuma JJ. Molecular epidemiology of Burkholderia species. Front Biosci. 2003;8 e55–67. doi: 10.2741/937. |
| 37. | St. John JA. Trojan horse L-selectin monocytes: a portal of Burkholderia pseudomallei entry into the brain. Virulence. 2017;8(6):611–612. doi: 10.1080/21505594.2016.1250997. |
| 38. | Currie BJ. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med. 2015;36(1):111–125. doi: 10.1055/s-0034-1398389. |
| 39. | Liu PJ, Chen YS, Lin HH, et al. Induction of mouse melioidosis with meningitis by CD11b+ phagocytic cells harboring intracellular B. pseudomallei as a Trojan horse. PLoS Negl Trop Dis. 2013;7(8):e2363. doi: 10.1371/journal.pntd.0002363. |
| 40. | Mahenthiralingam E, Baldwin A, Dowson CG. Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J Appl Microbiol. 2008;104(6):1539–1551. doi: 10.1201/9781003324010-3. |
| 41. | CDC. Clinical overview of melioidosis. Melioidosis. Updated May 6, 2024. https://www.cdc.gov/melioidosis/hcp/clinical-overview/index.html. Accessed July 19, 2024. |