ORIGINAL RESEARCH ARTICLE
Anesthetic and Analgesic Protocols in Spay-Neuter Clinics: A 2017 Survey of Practices and Preferences in the United States
Brendan S. Bergquist1*, Douglas dos Santos e Castro1, Virginia Aida-Ficken2, Nellie Goetz3 and Erik H. Hofmeister1
1Department of Clinical Sciences, College of Veterinary Medicine, Auburn University, Auburn, AL, USA; 2Department of Pathobiology, College of Veterinary Medicine, Auburn University, Auburn, AL, USA; 3College of Veterinary Medicine, University of Arizona, Oro Valley, AZ, USA
Abstract
Introduction: The advancement of spay-neuter procedures has been vital to the reduction of euthanized dogs and cats. Even though many spay-neuter clinics and shelters have adopted anesthesia protocols that align with best practices, there is currently little published data reporting trends among these clinics in the United States. The aim of this study was to provide data on the most commonly used high-quality, high-volume spay-neuter (HQHVSN) anesthetic/analgesic protocols.
Methods: In 2017, a voluntary, anonymous web-based survey was distributed to shelters/clinics through the HQHVSN veterinarians’ listserv and the Association of Shelter Veterinarians (ASV) listserv.
Results: One hundred and six facilities participated in the survey spanning 36 states in the United States. The most commonly used canine anesthesia premedication was acepromazine paired with an opioid, with acepromazine/hydromorphone and acepromazine/butorphanol representing 26% (24/91) and 23% (23/91) of the responses, respectively. Ketamine/midazolam was the most commonly used canine induction anesthetic representing 39% (35/91) of the responses. The most commonly used feline protocol was a total intramuscular anesthetic combination such as dexmedetomidine/ketamine/butorphanol (DKT) or Telazol/butorphanol/dexmedetomidine (TTDex) accounting for 39% (35/91) and 33% (30/91) of the responses, respectively. The majority of respondents administered an injectable nonsteroidal anti-inflammatory drug (NSAID) to their canine and feline patients at 63% (67/106) and 59% (63/106)), respectively. Only 26% (25/98) of respondents used to-go-home (TGH) medications, and only 40% (41/102) of respondents used local anesthetics. Overall protocol satisfaction was 86% (59/66).
Conclusion: This survey identified that across a wide range of spay-neuter clinics, there exists significant trends with regard to anesthetic and analgesic protocols. These results can be used as primary, historical data to which future studies can compare.
Keywords: HQHVSN, analgesic protocols, multimodal analgesia, spay-neuter anesthesia
Citation: Journal of Shelter Medicine and Community Animal Health 2024, 3: 105 - http://dx.doi.org/10.56771/jsmcah.v3.105
Copyright: © 2024 Brendan S. Bergquist et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 30 May 2024; Revised: 21 August 2024; Accepted: 13 September 2024; Published: 15 November 2024
Reviewers: Sara White, Simone Guerios
Correspondence: *Brendan S. Bergquist, 1130 Wire Rd, Auburn, AL 36849. Auburn University College of Veterinary Medicine. Email: bsb0018@auburn.edu
Competing interests and funding: The authors declare no potential conflicts of interest. The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
In recent decades, shelter medicine has seen remarkable advancements, primarily attributed to the adoption of best practices and a strong commitment to upholding high standards of animal welfare.1 The evolution of spay-neuter services has had a significant impact on the pet overpopulation problem and has encouraged progressive animal sheltering practices. Since its inception in the early 2000s, the Association of Shelter Veterinarians (ASV) has had a strong goal to advance spay/neuter programs. In the ASV’s 2016 Veterinary Medical Care Guidelines for Spay-Neuter Programs, the term high-quality, high-volume spay-neuter (HQHVSN) was defined.2 This term highlighted the importance of maintaining the highest quality individual patient care while being able to provide sterilization services to a large volume of animals. HQHVSN clinics have become vital to animal shelters themselves and the community. Notably, in the United States, annual estimated euthanasia rates of shelter animals have dropped from 22 to 24 million in the early 1970s to just under 1 million in 2019. Additionally, in 2023, estimated national reports indicated that 850,000 dogs and cats had non-live outcomes from animal sheltering organizations. The increasing number of facilities offering HQHVSN services has been a vital part of this overall decline.3,4
The field of shelter medicine has now become an integral component of veterinary education, significantly enhancing the confidence and knowledge of veterinary students.5–7 The challenge faced by shelter medicine programs is to deliver cost-effective, high-volume services while maintaining the highest standards of quality. In this unique context, shelter veterinarians require a comprehensive understanding of various disciplines, including epidemiology, internal medicine, diagnostic imaging, anesthesia, and surgery, among others.8 Of particular importance is a solid foundation in pharmacology, as this field often involves working with feral, stressed, or unhandled animals, which may necessitate chemical immobilization for diagnostic procedures and, in the case of HQHVSN programs, anesthesia administration.9,10
When developing and selecting anesthetic protocols for HQHVSN clinics and shelters, there are many factors to take into consideration, such as number and type of patients, size and experience of support staff, timing, ease of administration, cost, safety margin, and drug availability. Therefore, many protocols have been tailored to meet the needs of these programs. In practice, there is no single drug that encompasses all the necessary attributes: cost-effectiveness, ease of administration, minimal injection volume, rapid onset, prolonged effect, and the ability to provide muscle relaxation, hypnosis, analgesia, and hemodynamic stability.2,11,12 Nonetheless, there has been a growing shift in shelter medicine toward refining anesthetic protocols along with surgical techniques to adopt a more multimodal approach. Research based on a 6 year study from the University of Florida’s shelter medicine program demonstrated that this approach in a HQHVSN setting significantly reduced perioperative mortality rates and compares favorably with rates reported in private clinical practice.13
Shelter veterinarians have a range of pharmacological tools at their disposal, including alpha 2-adrenergic agonists, phenothiazines, opioids, dissociative anesthetics, phenolics, local anesthetics, anticholinergics, inhalant anesthetics, and nonsteroidal anti-inflammatory drugs (NSAIDs). This diverse array of drugs can be effectively integrated to create balanced anesthesia protocols suitable for high-volume spay-neuter procedures, as evidenced by various studies.14–16
There are many excellent resources available for shelter and HQHVSN veterinarians regarding anesthetic and analgesic protocols that remain in line with current accepted guidelines.11,17,18 However, it is unknown what protocols and strategies are used among HQHVSN clinics and shelters based on clinic qualities in the United States. The aim of this study was to collect and summarize HQHVSN anesthetic and analgesic protocol information.
Methods
Study design
Following approval from the Institutional Review Board at Midwestern University, a web-based survey was designed for the purpose of gathering data pertaining to anesthesia and analgesia protocols in spay-neuter clinics and shelters. The survey consisted of short, open-ended, multiple choice, and ranking questions. Two authors (N.G. and E.H.), one shelter medicine veterinarian, and one anesthesiologist designed the initial survey. It was pilot tested with six veterinarians and veterinary students for comprehension, and changes were made on the basis of feedback obtained. For the purposes of this survey, a ‘spay-neuter clinic’ was defined as a free-standing or mobile clinic that performs spays and neuters for shelters, rescues, community-owned, and/or privately-owned animals at least twice weekly. Animal shelters that focus solely on their own population of animals were also included. There was no requirement to complete a certain number of surgeries/day and clinics and shelters that perform spay-neuter surgeries on stray/outdoor/community cats only were eligible to participate. The survey was crafted and administered via an online platform (https://www.surveymonkey.com). It was distributed to these shelters/clinics through the HQHVSN veterinarians’ listserv and the ASV listserv. The survey remained accessible from August 2017 to December 2017. Participation in the survey was entirely voluntary and anonymous, and IP addresses were not evaluated. It was requested that only one representative from each clinic/shelter responds to the survey, and participants retained the flexibility to skip questions or discontinue the survey at their discretion. Respondents answered 22 questions with overall focuses being the facility geographical distribution and operational history, staffing details, surgical caseload and sex breakdown, current anesthetic/analgesic protocols, patient monitoring, protocol decision-making, and overall satisfaction (Appendix 1).
Statistical analysis
Incomplete surveys were not included in the analysis. Descriptive statistics stratified by institution type were generated for age of clinic (average, minimum, and maximum), number of veterinarians (median and mode), the number of surgeries performed per week (median, mode, minimum, and maximum), and the number of surgery days per week (median, mode, minimum, and maximum). Descriptive statistics stratified by institution type were also generated for the percentage of spays and neuters conducted yearly (mode, minimum, and maximum), the distribution of canine and feline surgeries conducted yearly (mode, minimum, and maximum), and ownership status of patients (mode, median, and average).
Participants provided percentages of surgeries with regard to dog surgeries, feline surgeries, and surgeries on owned, unowned, and feral animals. Normality was determined by the D’Agostino-Pearson method. Comparisons among institution types were made for yearly dog surgeries, yearly cat surgeries, and ownership of patients using an Analysis of Variance (ANOVA) with post-hoc Turkey testing or non-parametric Kruskal-Wallis test with post-hoc Dunn’s test. Six factors influencing protocol choice were ranked by participants and were categorized and reported as percentages. For each factor, the total counts for first place through sixth place were marked and then divided by the number of responses (n = 86) to obtain a percentage. When all categories were calculated, the category that had the highest percentage for the rank was identified. A comparison between satisfied participants and dissatisfied participants was further performed based on age of clinic, number of veterinarians in the clinic, number of surgeries performed per day, and number of surgery days per week using the Mann-Whitney U test. A chi-squared test was used to compare type of practice and protocol satisfaction. Significance was set at α = 0.05.
Results
Institution type & patient demographics
Data were collected from 106 facilities across 36 states in the United States. These facilities included 55 private nonprofit stationary clinics, 29 government institutions, nine private nonprofit mobile clinics, five academic institutions, five private for-profit stationary clinics, and three private for-profit mobile clinics (Fig. 1).
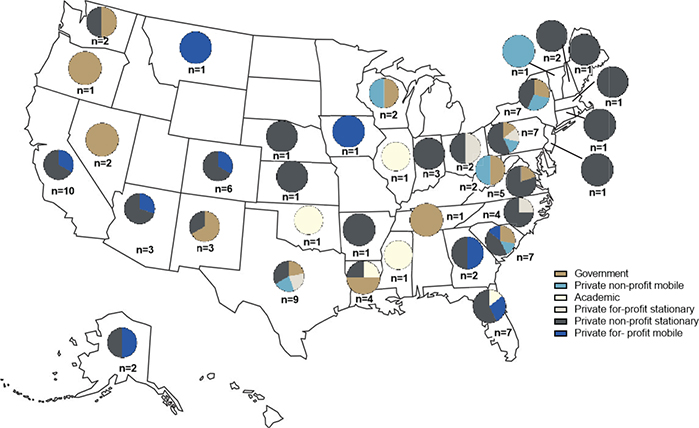
Figure 1. Geographic distribution of respondents according to type of practice.
On average, these facilities have been operational for approximately 13 years (minimum–maximum of 1.5–106). The staff included two veterinarians (1–3), who were supported by approximately three veterinarian technicians (2–4) and five veterinarian assistants (4–5). These clinics operated 5 days a week (1–7) and performed 31 (11–200) surgeries per day (Table 1). Regarding sex distribution, approximately 25% of the canine patients were males (5–60%) and approximately 25% were females (3–40%). Similarly, approximately 25% of feline patients were males (10–50%) and approximately 25% were females (10–60%). Approximately 50% of the dogs and cats were owned, and approximately 50% were unowned. Additionally, about 10% of the cats were feral.
Significant variations were observed in the distribution of canine and feline surgeries when comparing government institutions to private nonprofit mobile clinics. Government institutions exhibited a notably higher percentage of canine surgeries (P = 0.008), whereas private nonprofit mobile clinics showed a significantly higher percentage of feline surgeries (P = 0.006). However, no statistically significant differences were observed when comparing institution types regarding the annual frequency of spays and neuters.
A statistically significant difference in the percentage of surgeries conducted on owned dogs was observed when comparing government facilities to private-for-profit stationary clinics (P < 0.001), government to private nonprofit mobile clinics (P < 0.001), and government to private nonprofit stationary clinics (P < 0.001). Specifically, government facilities had notably fewer owned canine patients compared to all other types. Regarding owned cats, there was a significant difference among academic and private for-profit stationary (P = 0.049) and academic and private nonprofit mobile (P = 0.003) in which academic facilities performed a significantly lower number of surgeries on owned feline patients. Government facilities differed significantly compared to all groups except academic facilities (P = 0.001, < 0.001, < 0.001, and 0.029) in which government facilities performed significantly fewer surgeries on owned cats.
Anesthetic protocols
There was a diverse range of anesthetic protocols used for canine patients. The most common premedication protocol was the pairing of acepromazine with hydromorphone (24/91; 26%), closely followed by acepromazine with butorphanol (23/91; 23%). The acepromazine and hydromorphone combination was widely favored in both private for-profit and nonprofit stationary clinics. In academic institutions, acepromazine was frequently paired with hydromorphone, and additional options included butorphanol, carprofen, atropine, and meloxicam as premedication choices. Conversely, government institutions predominantly used acepromazine and butorphanol as their preferred protocol. During the induction phase, the most commonly used drugs were ketamine/midazolam (35/91; 39%), with Telazol, butorphanol, and dexmedetomidine (TTDex) (23/91; 25%) and Telazol (11/91; 12%) following closely behind (Table 2). For maintenance, the majority of responders (69/91; 76%) indicated that no additional drugs were needed for maintenance, with isoflurane being the second most commonly mentioned option at 24% (22/91).
Regarding anesthetic protocols reported for cats, a majority of respondents did not indicate that a premedication was given (46/91; 50%). Acepromazine alone, hydromorphone alone, a combination of acepromazine and butorphanol, and a combination of buprenorphine and robenacoxib were all employed at an equal frequency of 7.7% (7/91). The most common induction protocol for cats was dexmedetomidine, ketamine, and butorphanol (DKT), accounting for approximately 38% (35/91) of cases, followed closely by TTDex at 33% (30/91) (Table 3). Concerning maintenance, a significant majority of responders (73/91; 80%) indicated that no additional drugs were required, and only 20% (18/91) mentioned the use of isoflurane.
Analgesia
Sixty-three percent (67/106) of canine patients undergoing surgery received an injectable NSAID. Among the NSAIDs used, meloxicam was the most frequently administered (36/67; 54%), followed by carprofen (23/67; 34%), ketoprofen (5/67; 7.5%), meloxicam, and/or carprofen (2/67; 3.0%), and finally meloxicam and/or ketoprofen (1/67; 1.5%). With respect to clinic types, meloxicam was used most frequently by academic (2/3; 67%), government (9/15; 60%), and private nonprofit stationary facilities (23/41; 56%). Carprofen was used most frequently by private for-profit mobile (1/1; 100%), private nonprofit mobile (2/3; 67%), and private for-profit stationary facilities (2/4; 50%). The two types of facilities that used both carprofen and meloxicam were private for-profit stationary (1/4; 25%) and private nonprofit stationary (1/41; 2.4%) (Table 4). Regarding the timing of NSAID administration in canine patients, 37% (25/67) gave no indication, followed by 36% (24/67) administering postoperatively, 12% (8/67) administering preoperatively, 10.5% (7/67) administering at induction, 3.0% (2/67) administering intraoperatively, and 1.5% (1/67) administering pre- and/or postoperatively.
| NSAID | Overall | Academic* | Government | Private for-profit stationary | Private non-profit mobile | Private non-profit stationary | Private for-profit mobile** | ||||||||
| Canine (n = 67) | Feline (n = 63) | Canine (n = 3) | Feline (n = 3) | Canine (n = 15) | Feline (n = 15) | Canine (n = 4) | Feline (n = 4) | Canine (n = 3) | Feline (n = 2) | Canine (n = 41) | Feline (n = 38) | Canine (n = 1) | Feline (n = 1) | ||
| Injectable NSAID | Carprofen | 34 | 1.6 | 3.3 | 0 | 27 | 0 | 50 | 0 | 67 | 0 | 32 | 2.6 | 100 | 0 |
| Ketoprofen | 7.5 | 1.6 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 7.3 | 2.6 | 0 | 0 | |
| Meloxicam | 54 | 95 | 67 | 100 | 60 | 100 | 25 | 100 | 33 | 100 | 56 | 92 | 0 | 100 | |
| Carprofen, Meloxicam | 3.0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 2.4 | 0 | 0 | 0 | |
| Ketoprofen, Meloxicam | 1.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.4 | 0 | 0 | 0 | |
| Robenacoxib | 0 | 1.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.6 | 0 | 0 | |
| Additional Medication | Canine (n = 25) | Feline (n = 15) | Canine (n = 2) | Feline (n = 1) | Canine (n = 3) | Feline (n = 3) | Canine (n = 3) | Feline (n = 3) | Canine (n = 16) | Feline (n = 16) | Canine (n = 1) | ||||
| To-Go-Home Medication | Carprofen | 44 | 0 | 100 | 0 | 33 | 0 | 33 | 0 | 38 | 0 | 100 | |||
| Meloxicam | 16 | 27 | 0 | 100 | 0 | 0 | 0 | 0 | 25 | 30 | 0 | ||||
| Tramadol | 16 | 0 | 0 | 0 | 0 | 0 | 67 | 0 | 13 | 0 | 0 | ||||
| Firocoxib | 0 | 0 | 0 | 0 | 0 | 33 | 0 | 0 | 0 | 0 | 0 | ||||
| Trazadone | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Gabapentin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Buprenorphine | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 10 | 0 | ||||
| Buprenorphine SR | 0 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 0 | ||||
| Robenacoxib | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | ||||
| Carprofen, Meloxicam | 12 | 0 | 0 | 0 | 33 | 0 | 0 | 0 | 13 | 0 | 0 | ||||
| Buprenorphine, Robenacoxib | 0 | 6.7 | 0 | 0 | 0 | 33 | 0 | 0 | 0 | 0 | 0 | ||||
| Carprofen, Meloxicam, Firocoxib | 4.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6.3 | 0 | 0 | ||||
| Carprofen, Tramadol | 4.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6.3 | 0 | 0 | ||||
| Gabapentin | 0 | 6.7 | 0 | 0 | 0 | 33 | 0 | 0 | 0 | 0 | 0 | ||||
| Carprofen, Trazadone, Gabapentin | 4.0 | 0 | 0 | 0 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| *No Academic facilities used TGH medications. | |||||||||||||||
| **Private for-profit mobile institutions did not prescribe TGH medications to feline patients. | |||||||||||||||
For feline patients, NSAIDs were employed in 59% (63/106) of the facilities, with meloxicam being the preferred choice at 95% (60/63). Specifically, academic, government, private for-profit stationary, private nonprofit mobile, and private for-profit mobile clinics exclusively administered meloxicam. Notably, robenacoxib, carprofen, and ketoprofen were occasionally used in private nonprofit stationary clinics, but meloxicam remained the dominant choice at 92% (35/38) usage in these facilities (Table 4). Regarding the timing of NSAID administration in feline patients, 38% (24/63) gave no indication, followed by 33% (21/63) administering postoperatively, 13% (8/63) administering preoperatively, 11% (7/63) administering at induction, 3% (2/63) administering intraoperatively, and 2% (1/63) administering pre- and/or postoperatively. Thirty-five percent (37/106) of the participants reported no implementation of NSAIDs in spay-neuter protocols. A variety of factors were reported to justify the absence of NSAID administration, including pediatric patients, sick conditions, dehydration, potential kidney disease, and oral administration as sufficient for analgesia postoperatively. To-go-home (TGH) medications were only used 26% (25/98) of the time, with carprofen being the most commonly used in canine patients at 44% (11/25) and meloxicam being the most commonly used in feline patients at 27% (4/15) (Table 4).
There were six justifications for not prescribing TGH medications to patients: TGH deemed unnecessary (23/73; 32%), dispensing limitations (7/73; 10%), cost alone (4/73; 5.5%), a combination of cost and dispensing limitations (5/73; 6.9%), a combination of not necessary and dispensing limitations (2/73; 2.7%), and a combination of not necessary, cost, and dispensing limitations (2/73; 2.7%). Forty-one percent (30/73) of participants who reported not using TGH medications did not indicate a justification.
Only 40% (41/102) of the survey participants described using local anesthetic techniques during spay and neuter procedures. When it came to intratesticular blocks, lidocaine only was used 34% (14/41) of the time, and bupivacaine alone was used 10% (4/41) of the time. Lidocaine and/or bupivacaine was used 34% (14/41) of the time, and 22% (9/41) gave no indication of using either. For splash blocks, lidocaine and/or bupivacaine was used 29% (12/41) of the time, bupivacaine alone was used 24% (10/41) of the time, and 46% (19/41) of respondents gave no indication of using either. Respondents who did not use local anesthetic blocks during spay and neuter procedures cited reasons such as time constraints, staff availability, and the perception that such techniques were unnecessary.
Monitoring
Eighty-six percent (80/93) of respondents used both manual and electronic anesthesia monitoring. Specifically, 50% (46/93) of respondents monitored patients using a combination of pulse oximetry, heart and respiratory rate palpation/auscultation, and jaw tone/eye position. Nine percent (8/93) of respondents monitored patients using a combination of pulse oximetry, EtCO2, ECG, heart and respiratory rate palpation/auscultation, and jaw tone/eye position. Ten percent (9/93) of respondents relied solely heart and respiratory rate palpation/auscultation, while 2.2% (2/93) of respondents used only pulse oximetry. Other combinations about how animals were monitored represented 26% (24/93) of responses. No answer was given by 4.3% (4/93) of respondents.
Overall, the responsibility for monitoring patients during anesthesia fell to technicians or assistants in 62% (58/93) of cases, and in 26% (24/93) of instances, the surgeon was also involved. The surgeon alone was responsible for monitoring only 1.1% (1/93) of the time. The combination of a technician/assistant and a volunteer was also indicated only 1.1% (1/93) of the time. No answer was given by 10% (9/93) of respondents. In government facilities, 86% (18/21) reported relying solely on veterinary technicians for monitoring, with the remaining 14% (3/21) using both surgeons and technicians. Private for-profit stationary clinics exclusively used technicians for anesthesia monitoring, while private nonprofit mobile and stationary clinics employed both technicians and veterinary surgeons in monitoring.
Protocol choice
Nine factors influenced current anesthetic protocol choice. These factors included: previous experience with the protocol of choice alone (20/69; 29%), scientific literature alone (5/69; 7.3%), guidance from the Humane Alliance/American Society for the Prevention of Cruelty to Animals (ASPCA) Spay/Neuter Alliance alone (5/69; 7.3%), difficulty obtaining schedule II opioids alone (4/69; 5.8%), cost alone (1/69; 1.5%), recommendation from other shelter veterinarian alone (2/69; 2.9%), ease of administration alone (2/69; 2.9%), safety alone (1/69; 1.5%), and recommendations by an anesthesiologist alone (1/69; 1.5%). Forty-one percent (28/69) of respondents indicated that multiple factors influenced their decision. Respondents ranked six factors in deciding whether to include or change an element in a protocol. The rankings were recommendations from peer-reviewed literature (1st), recommendations from HQHVSN training facilities (2nd), cost (3rd), acceptance by other HQHVSN practitioners (4th), veterinary-based information services (5th), and acceptance by full-service practitioners in the area (6th) (Fig. 2).
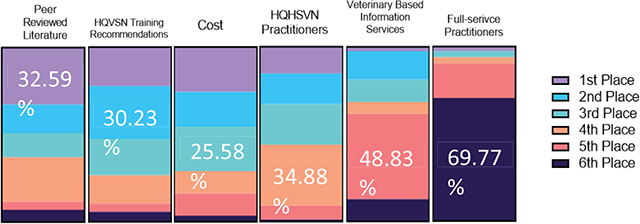
Figure 2. Illustration of rankings per category.
Protocol satisfaction
The majority of participants (59/66; 86%) were satisfied with their current anesthetic protocol. All academic institutions, private for-profit stationary, and private for-profit mobile clinics were satisfied with their current protocols. There was no significant difference between dissatisfied clinics and satisfied clinics with respect to age of clinic (P = 0.733), number of veterinarians on staff (P = 0.095), number of surgeries per day (P = 0.775), or number of surgeries per week (P = 0.168). There was no significance detected between the type of institution and frequency or tendency to be satisfied with the protocol (P values ranged from 0.27 to > 0.99).
Difficulty of getting schedule II opioids alone encompassed a rather small percentage of total responses (4/69; 5.8%) for reasons in choosing a protocol. Interestingly, this reason was indicated by clinics dissatisfied with their protocol as the sole barrier to change at 56% (5/9). Other reasons to change protocol and/or barriers to change included wanting better multimodal analgesia (1/9; 11%), a combination of better multimodal analgesia and more reversible anesthesia (1/9; 11%), a combination of cost and difficulty using schedule II opioids (1/9; 11%), and a combination of better multimodal analgesia and staff compliance (1/9; 11%).
Discussion
The aim of this study was to collect and summarize HQHVSN anesthetic and analgesic protocol information. The results support this aim and show that there is overall consensus (59/66; 86%) with regard to satisfaction of current protocols as well as provide unique insight into significant trends that exist surrounding protocol decision-making and selection of specific medications.
The factors that most influenced anesthetic protocol choice were previous experience with the protocol of choice (38/69; 52%), scientific literature (20/69; 29%), and guidance from the Humane Alliance/ASPCA Spay/Neuter Alliance (14/69; 20%). What is particularly interesting is that when participants were asked to rank the importance of implementing a protocol, peer reviewed literature (28/86; 33%) and HQHVSN training recommendations (26/86; 30%) received the 1st and 2nd place rankings, respectively. These results point to the fact that the increased amount of HQHVSN resources and training opportunities that are available to veterinary students and veterinarians have impacted anesthetic protocol trends that align more with a high-volume surgical setting. Cost still remained an important factor among respondents.
With respect to anesthesia protocols in feline patients, most respondents indicated using DKT or TTDex for induction at 38% (35/91) and 33% (30/91), respectively. One of the major benefits of using these protocols in a HQHVSN setting is the ability to use it as a total injectable anesthetic combination. TTDex when used this way provides a surgical plane of anesthesia for 30 to 40 min with a very rapid onset action of about 3 to 5 min after intramuscular injection.11 Veterinarians and veterinary students in these settings have been using safe, reliable HQHVSN surgical techniques such as the feline ovarian pedicle tie, thus producing shortened surgical and anesthetic times.19,20 It is not uncommon for trained HQHVSN surgeons to have their feline ovariohysterectomy times be around 4–5 min from open to close. By taking a total injectable anesthesia approach, it eliminates the need for intravenous catheter placement, endotracheal tube intubation, and many times maintenance on inhalant anesthesia, which can prove to be quite time consuming in this setting with a large volume of animals and a relatively short surgical time. A majority of respondents indicated that no additional drugs were needed for maintenance anesthesia (73/91; 80%). While with a total injectable anesthetic approach, this may be true, some of these responses may have been attributed to how the survey question was written, with inhalant anesthetics not being asked about specifically. It is also important to note that many of these operations have community cat management programs in place that perform trap-neuter-release (TNR) on unowned, free-roaming cats. Many of these cats are not able to be handled, making these anesthetic protocols ideal with regard to safety and administration. Therefore, these total injectable anesthetic combinations are highly desirable in these situations due to their wide safety margin as well as from an efficiency standpoint.
In canine patients, there was a significant trend with a majority of respondents using either acepromazine/hydromorphone (24/91; 26%) or acepromazine/butorphanol (23/91; 23%) as a premedication. This combination of using a phenothiazine tranquilizer such as acepromazine with that of an opioid analgesic agent such as hydromorphone or butorphanol has been termed neuroleptanalgesia and has been used widely in veterinary practice. This combination provides balanced anesthesia through multimodal analgesia as well as sparing effects on the requirements of other anesthetics.21 For induction, a significant majority of respondents indicated using the combination of ketamine and midazolam (35/91; 39%). Using a dissociative anesthetic such as ketamine in combination with a benzodiazepine such as midazolam or diazepam allows for a reliable, safe induction of anesthesia in healthy canine patients, similar to that of propofol.22 While propofol has been widely used as an induction anesthetic in veterinary medicine, specifically general practice, in this study, respondents only indicated its use 2.2% (2/91) of the time. This finding is likely attributed to a few different factors. Due to the aforementioned shortened surgical and anesthetic times in a HQHVSN setting, it is a common practice to not place a catheter in these patients. It is not ideal to administer propofol without an intravenous catheter due to the larger volume as well as the fact that it is administered to effect. On the other hand, midazolam can be easily combined with ketamine in a single syringe and administered intravenously without a catheter. This combination is also likely preferred due to the cost per patient. For example, in a 25 kg dog, clinic cost of 1.25 mL of ketamine (100 mg/mL) at a dose of 5 mg/kg would be $0.93, and 2.25 mL of midazolam (5 mg/mL) at a dose of 0.45 mg/kg would be $0.42. On the other hand, in the same dog, clinic cost of 10–15 mL of propofol at a dose of 4–6 mg/kg would be $6.10 to $9.15 (Teri B. Etheredge, Pharm.D., Auburn University College of Veterinary Medicine, email communication, April 18, 2024). With regard to maintenance, most respondents indicated that no additional drugs were needed (73/91; 80%). While some of these responses could have been attributed to a misunderstanding of the survey question, it is possible that a total injectable anesthesia protocol, particularly including Telazol, would be sufficient to accomplish surgery on canine patients.
In veterinary medicine, perioperative pain management is a vital component to the clinical success of our patients. When addressing this, basic principles such as preemptive use of analgesics, applying a multimodal approach, providing overlapping analgesia, and matching the provision of analgesia to the degree of surgery are all extremely important regardless of the surgical setting.23 Specifically, this study evaluated the use of injectable NSAIDs, TGH medications, and local anesthetics in these HQHVSN settings. The combination of these analgesics with that of opioids used in premedication and total injectable anesthetic protocols helps address these principles. The results showed that, while a majority of respondents indicated the use of injectable NSAIDs in their canine (67/106; 63%) and feline (63/106; 59%) patients, more than a third of clinics did not use NSAIDs. Furthermore, the majority of respondents indicated not using TGH medication or local anesthetics in their current protocols. Some of these trends do not appear to be in strong alignment with the basic principles of perioperative pain management as previously discussed. These observations are likely attributed to a number of factors. When considering the provision of analgesia to the degree of surgery, it has been observed that things like increased tissue trauma, nerve damage, and more invasive/longer surgical procedures have been related to more pain. Generally, experienced HQHVSN surgeons tend to cause minimal tissue trauma and have short surgical times compared to a more novice surgeon. Additionally, spay-neuter surgeries tend to be somewhat less invasive than other surgical procedures. Some respondents who indicated not using TGH medications felt that they were not needed. The use of an injectable NSAID at the time of surgery will provide some overlapping/continuous analgesia typically for a period of 12–24 h. The use of additional TGH medications may be indicated; however, in these settings, there are factors such as limited patient follow-up and postoperative pain assessment that can inhibit identifying the need. Additionally, many of the owned animals undergoing spay-neuter surgeries at these facilities tend to come from lower income families where the cost of additional medications may be prohibitive. Finally, given that the data were collected in 2017, it is possible that shifts are occurring such that clinics are slowly adopting more NSAIDs or local anesthetic techniques. Subsequent studies would be necessary to document if this is occurring.
Regarding the use of local/regional anesthetics, such as intratesticular blocks and incisional splash blocks, respondents only indicated the use (41/102; 40%) of the time. This is a pharmacologic modality that is still somewhat new to these spay-neuter settings. Understanding, acceptance, and practical application appear to be the biggest barriers to adding this into current protocols. However, many of the current HQHVSN training facilities have incorporated this into their protocols. As veterinarians and veterinary students continue to train at this level, it is expected that the understanding and use of this technique will continue to rise.
Limitations
One limitation of this study is that these data were collected in 2017, and some changes in anesthetic practice have been observed by the authors (e.g. wider adoption of local anesthetic techniques). Furthermore, some drugs currently available (e.g. alfaxalone and transdermal buprenorphine) were not available or widely used at the time of the survey collection. Therefore, these data cannot be used to document current trends in HQHVSN clinics. This study should be repeated regularly to compare the most current trends with this primary, historical data. Another important limitation of this study was the study design. Some of the questions in the survey were presented in a free-response format with multiple queries per question, and this resulted in a lot of non-responses. Should this survey be repeated in the future, these questions can be formatted differently to help decrease this ambiguity. Additionally, these results may not be a true representation of HQHVSN clinics in the USA because of the platforms that were used for distribution of the survey, thus not making it completely random. While this is difficult to achieve, the authors would like to acknowledge this potential bias in the clinics samples as a limitation.
Conclusion
This survey identified historical practices with regard to anesthetic and analgesic protocols in HQHVSN clinics. These practices are likely due to the increase in opportunities made available to veterinary students at HQHVSN training facilities and academic institutions that focus on continuous refinement of anesthesia protocols based on the highest standard of quality. As these opportunities continue and as shelter medicine evolves, these results can be used as primary, historical data to which future studies can compare.
Authors’ contributions
Brendan S. Bergquist: Conceptualization, Data Curation, Writing-Original Draft; Douglas dos Santos e Castro: Conceptualization, Validation, Writing – Review & Editing; Virginia Aida-Ficken: Data Curation, Formal Analysis, Writing – Review & Editing; Nellie Goetz: Conceptualization, Methodology, Writing – Review and Editing; Erik H. Hofmeister: Conceptualization, Supervision, Formal Analysis, Writing – Review and Editing.
Acknowledgments
The authors thank Teri B. Etheredge, Pharm.D., Director of Pharmacy, Auburn University College of Veterinary Medicine for providing cost analysis of specific anesthetic drugs.
References
| 1. | Rowan A, Kartal T. Dog Population & Dog Sheltering Trends in the United States of America. Animals (Basel). 2018;8(5):68. doi: 10.3390/ani8050068 |
| 2. | Association of Shelter Veterinarians’ Veterinary Task Force to Advance Spay-Neuter, Griffin B, Bushby PA, et al. The Association of Shelter Veterinarians’ 2016 Veterinary Medical Care Guidelines for Spay-Neuter Programs. J Am Vet Med Assoc. 2016;249(2):165–188. doi: 10.2460/javma.249.2.165 |
| 3. | Bushby PA. High-Quality, High-Volume Spay-Neuter: Access to Care and the Challenge to Private Practitioners. J Feline Med Surg. 2020;22(3):208–215. doi: 10.1177/1098612X20903600 |
| 4. | Intake and Outcome Data Analysis, Full Year 2023. Shelter Animals Count; 2024. Accessed Jul 31, 2024. https://www.shelteranimalscount.org/wp-content/uploads/2024/01/Full-Year-2023-Report.pdf |
| 5. | Shivley JM, Brookshire WC, Bushby PA, Woodruff KA. Clinically Prepared Veterinary Students: Enhancing Veterinary Student Hands-on Experiences and Supporting Hospital Caseload Using Shelter Medicine Program. Front Vet Sci. 2018;5:95. doi: 10.3389/fvets.2018.00095 |
| 6. | Hedge ZN, Bossong F, Gordon-Ross PN, Kovacs SJ. Exploring the Effects of Participation in a Shelter Medicine Externship on Student Knowledge and Self-Confidence. J Vet Med Educ. 2019;46(1):4–13. doi: 10.3138/jvme.0417-056r |
| 7. | Powell L, Reinhard CL, Serpell J, Watson B. A Survey of Veterinary Student and Veterinarian Perceptions of Shelter Medicine Employment. J Vet Med Educ. 2023;50(1):27–52. doi: 10.3138/jvme-2021-0112 |
| 8. | Foley JE. The Educational Discipline of Shelter Medicine. J Vet Med Educ. 2003;30(4):379–382. doi: 10.3138/jvme.30.4.379 |
| 9. | Cistola AM, Golder FJ, Centonze LA, McKay LW, Levy JK. Anesthetic and Physiologic Effects of Tiletamine, Zolazepam, Ketamine, and Xylazine Combination (TKX) in Feral Cats Undergoing Surgical Sterilization. J Feline Med Surg. 2004;6(5):297–303. doi: 10.1016/j.jfms.2003.11.004 |
| 10. | Williams LS, Levy JK, Robertson SA, Cistola AM, Centonze LA. Use of the Anesthetic Combination of Tiletamine, Zolazepam, Ketamine, and Xylazine for Neutering Feral Cats. J Am Vet Med Assoc. 2002;220(10):1491–1495. doi: 10.2460/javma.2002.220.1491 |
| 11. | Ko JC, Berman AG. Anesthesia in Shelter Medicine. Top Companion Anim Med. 2010;25(2):92–97. doi: 10.1053/j.tcam.2010.03.001 |
| 12. | Grubb T, Sager J, Gaynor JS, et al. 2020 AAHA Anesthesia and Monitoring Guidelines for Dogs and Cats. J Am Anim Hosp Assoc. 2020;56(2):59–82. doi: 10.5326/JAAHA-MS-7055 |
| 13. | Levy JK, Bard KM, Tucker SJ, Diskant PD, Dingman PA. Perioperative Mortality in Cats and Dogs Undergoing Spay or Castration at a High-Volume Clinic. Vet J. 2017;224:11–15. doi: 10.1016/j.tvjl.2017.05.013 |
| 14. | Sylvestre FR, Monteiro BP, Simard MJ, Steagall PV. Anesthetic Effects of Ketamine-Medetomidine-Hydromorphone in Dogs During High-Quality, High-Volume Surgical Sterilization Program Under Field Conditions. Vet Anaesth Analg. 2020;47(6):789–792. doi: 10.1016/j.vaa.2020.08.001 |
| 15. | Moser KL, Hasiuk MM, Armstrong T, Gunn M, Pang DS. A Randomized Clinical Trial Comparing Butorphanol and Buprenorphine within a Multimodal Analgesic Protocol in Cats Undergoing Orchiectomy. J Feline Med Surg. 2020;22(8):760–767. doi: 10.1177/1098612X19886132 |
| 16. | Kushnir Y, Toledano N, Cohen L, Bdolah-Abram T, Shilo-Benjamini Y. Intratesticular and Incisional Line Infiltration with Ropivacaine for Castration in Medetomidine-Butorphanol-Midazolam Sedated Dogs. Vet Anaesth Analg. 2017;44(2): 346–355. doi: 10.1016/j.vaa.2016.03.007 |
| 17. | Drug Protocols for Spay/Neuter Clinics. American Society for the Prevention of Cruelty to Animals; 2024. Accessed Jul 31, 2024. https://www.aspcapro.org/resource/drug-protocols-spayneuter-clinics |
| 18. | White S. High-Quality, High-Volume Spay and Neuter and Other Shelter Surgeries. Wiley-Blackwell Publishing; 2019. |
| 19. | Miller KP, Rekers W, Ellis K, Ellingsen K, Milovancev M. Pedicle Ties Provide a Rapid and Safe Method for Feline Ovariohysterectomy. J Feline Med Surg. 2016;18(2):160–164. doi: 10.1177/1098612X15576589 |
| 20. | Rigdon-Brestle K, Accornero VH, Amtower M, Slater MR. Retrospective Review Reveals Few Complications of Ovarian Pedicle Tie in 15,927 Cats Undergoing Ovariohysterectomy at a Large HQHVSN Clinic and Training Facility in the United States: 2017–2018. J Am Vet Med Assoc. 2022;260(S2):S28–S35. doi: 10.2460/javma.21.09.0405 |
| 21. | Monteiro ER, Figueroa CD, Choma JC, Campagnol D, Bettini CM. Effects of Methadone, Alone or in Combination with Acepromazine or Xylazine, on Sedation and Physiologic Values in Dogs. Vet Anaesth Analg. 2008;35(6):519–527. doi: 10.1111/j.1467-2995.2008.00412.x |
| 22. | Ferreira JP, Dzikit TB, Zeiler GE, et al. Anaesthetic Induction and Recovery Characteristics of a Diazepam-Ketamine Combination Compared with Propofol in Dogs. J S Afr Vet Assoc. 2015;86(1):1258. doi: 10.4102/jsava.v86i1.1258 |
| 23. | Gruen ME, Lascelles BDX, Colleran E, et al. 2022 AAHA Pain Management Guidelines for Dogs and Cats. J Am Anim Hosp Assoc. 2022;58(2):55–76. doi: 10.5326/JAAHA-MS-7292 |
Appendix 1. Questionnaire
- What state is your spay/neuter clinic located in? For those with multiple locations, please select the main clinic location.
- How long has the spay/neuter clinic been in operation?
- How many full-time veterinarians perform spay/neuter services?
- How many veterinarians are in surgery at the same time?
- How many support staff/para staff are employed per surgical doctor?
- What is the average number of spay/neuter surgeries performed per day?
- How many days per week are spay/neuter surgeries performed?
- What is the approximate species and sex breakdown of surgeries annually?
a. Dogs ____% Spays ____% Neuters ____ %
b. Cats ____% Spays ____% Neuters ____ %
i. Owned ____% Feral/Free-roaming/Community ____%
- Do you routinely use injectable NSAIDs for your all of your spay/neuter patients?
a. If yes, which NSAID, what dose, and when is it given?
b. If no, which group(s) do you not use them in and what is your reason for not using them in that group?
- Do you or your staff routinely perform local blocks or splash blocks on spay/neuter patients?
a. If yes, list blocks and who is responsible for performing them?
b. If no, what is your reason for not performing them?
- Do you routinely prescribe pain medication for patients postoperatively?
a. If yes, what medication, dose, and duration, and what is your reason for prescribing them routinely?
i. Does this differ by ownership status?
1. If yes, what is the reason for the differential prescribing?
b. If no, what is your reason for not prescribing them routinely?
- Analgesic protocols: Please detail any additional analgesics you routinely utilize in your protocol (not including NSAIDs, postoperative pain medications, or local blocks), including drug dosages and differences by species or ownership status.
- Anesthetic protocols: Please provide your current anesthetic protocols (preanesthetics/premeds, induction drugs, and maintenance drugs), including drug dosages and differences by species or ownership status.
- How are anesthetized animals monitored?
a. Manual monitoring only
b. Combination of manual and electronic monitoring
c. Electronic monitoring only
- If manual monitoring is employed, please describe:
- If electronic monitoring is employed, please describe:
- Who is responsible for making decisions relating to anesthetic/analgesic protocols? Include all that apply, in order of who has the most responsibility
a. Lead Veterinarian/Medical Director
b. Staff Veterinarian(s)
c. Veterinary technicians
d. Veterinary assistants
e. Executive Director/CEO
- How did you decide on your current protocol?
- When did you implement your current protocol?
- How do you perform cost analyses before switching part or all of your anesthetic/analgesic protocol?
- How important are each of the following in deciding whether to include or change an element in an anesthetic/analgesic protocol? Please order by importance.
a. Cost
b. Acceptability with other HQHVSN practitioners
c. Acceptability with full-service practitioners in area
d. Recommendations from HQHVSN training facilities (i.e. Humane Alliance, Emancipet, etc.)
e. Recommendations from veterinary-based information services (e.g. VIN, webinars, etc.)
f. Recommendations based on peer-reviewed literature
- Are you satisfied with your current anesthetic/analgesic protocol?
a. If not, what would you like to change, and what are the barriers to changing?